November 25, 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -

Thanksgiving Weather Louisville, Kentucky
Thanksgiving Weather Louisville, Kentucky Highest temperature: 73° on November 26, 1896 Highest daily average temperature: 65.5° on November 26, 1896 Lowest temperature: 8° on November 27, 1930 Lowest average daily temperature: 14.5° on November 27, 1930 Wettest: 2.19” on November 25, 2010 Snowiest: 1.2” on November 24, 1938 Deepest Snow Cover: 1” on November 28, 1929; November 27, 1930; and November 25, 1971 High Temp Low Temp Precipitation Snowfall Snow Depth November 28, 1872 39 19 0 November 27, 1873 49 34 0 November 26, 1874 46 25 0 November 25, 1875 52 27 0.05 November 30, 1876 33 24 0.03 November 29, 1877 32 23 0 November 28, 1878 45 36 0 November 27, 1879 65 56 1 November 25, 1880 31 24 0.23 November 24, 1881 27 19 T November 30, 1882 42 32 0 November 29, 1883 56 37 0 November 27, 1884 60 45 0 0 November 26, 1885 49 38 0 0 November 25, 1886 39 25 T T November 24, 1887 60 48 0.32 0 November 29, 1888 42 37 0.01 0 November 28, 1889 37 23 0.06 0.1 November 27, 1890 51 27 0 0 November 26, 1891 52 37 T 0 November 24, 1892 38 16 0 0 November 30, 1893 49 38 0 0 November 29, 1894 40 23 T 0 November 28, 1895 55 28 0 0 November 26, 1896 73 58 0 0 November 25, 1897 56 47 0.23 0 November 24, 1898 35 21 0 0 November 30, 1899 66 48 0 0 November 29, 1900 39 31 0 0 0 High Temp Low Temp Precipitation Snowfall Snow Depth November 28, 1901 42 27 0 0 0 November 27, 1902 37 32 T T 0 November 26, 1903 29 19 0 0 0 November 24, 1904 66 35 0 0 0 November 30, 1905 30 22 0 0 0 November 29, 1906 43 32 0 0 0 November 28, 1907 51 36 0 0 0 November 26, 1908 -
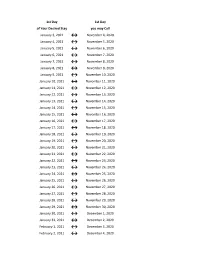
Flex Dates.Xlsx
1st Day 1st Day of Your Desired Stay you may Call January 3, 2021 ↔ November 4, 2020 January 4, 2021 ↔ November 5, 2020 January 5, 2021 ↔ November 6, 2020 January 6, 2021 ↔ November 7, 2020 January 7, 2021 ↔ November 8, 2020 January 8, 2021 ↔ November 9, 2020 January 9, 2021 ↔ November 10, 2020 January 10, 2021 ↔ November 11, 2020 January 11, 2021 ↔ November 12, 2020 January 12, 2021 ↔ November 13, 2020 January 13, 2021 ↔ November 14, 2020 January 14, 2021 ↔ November 15, 2020 January 15, 2021 ↔ November 16, 2020 January 16, 2021 ↔ November 17, 2020 January 17, 2021 ↔ November 18, 2020 January 18, 2021 ↔ November 19, 2020 January 19, 2021 ↔ November 20, 2020 January 20, 2021 ↔ November 21, 2020 January 21, 2021 ↔ November 22, 2020 January 22, 2021 ↔ November 23, 2020 January 23, 2021 ↔ November 24, 2020 January 24, 2021 ↔ November 25, 2020 January 25, 2021 ↔ November 26, 2020 January 26, 2021 ↔ November 27, 2020 January 27, 2021 ↔ November 28, 2020 January 28, 2021 ↔ November 29, 2020 January 29, 2021 ↔ November 30, 2020 January 30, 2021 ↔ December 1, 2020 January 31, 2021 ↔ December 2, 2020 February 1, 2021 ↔ December 3, 2020 February 2, 2021 ↔ December 4, 2020 1st Day 1st Day of Your Desired Stay you may Call February 3, 2021 ↔ December 5, 2020 February 4, 2021 ↔ December 6, 2020 February 5, 2021 ↔ December 7, 2020 February 6, 2021 ↔ December 8, 2020 February 7, 2021 ↔ December 9, 2020 February 8, 2021 ↔ December 10, 2020 February 9, 2021 ↔ December 11, 2020 February 10, 2021 ↔ December 12, 2020 February 11, 2021 ↔ December 13, 2020 -
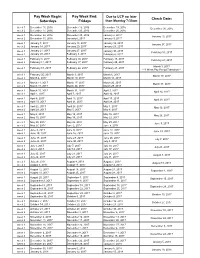
Pay Week Begin: Saturdays Pay Week End: Fridays Check Date
Pay Week Begin: Pay Week End: Due to UCP no later Check Date: Saturdays Fridays than Monday 7:30am week 1 December 10, 2016 December 16, 2016 December 19, 2016 December 30, 2016 week 2 December 17, 2016 December 23, 2016 December 26, 2016 week 1 December 24, 2016 December 30, 2016 January 2, 2017 January 13, 2017 week 2 December 31, 2016 January 6, 2017 January 9, 2017 week 1 January 7, 2017 January 13, 2017 January 16, 2017 January 27, 2017 week 2 January 14, 2017 January 20, 2017 January 23, 2017 January 21, 2017 January 27, 2017 week 1 January 30, 2017 February 10, 2017 week 2 January 28, 2017 February 3, 2017 February 6, 2017 week 1 February 4, 2017 February 10, 2017 February 13, 2017 February 24, 2017 week 2 February 11, 2017 February 17, 2017 February 20, 2017 March 3, 2017 week 1 February 18, 2017 February 24, 2017 February 27, 2017 ***1 Week Pay Period Transition*** week 1 February 25, 2017 March 3, 2017 March 6, 2017 March 17, 2017 week 2 March 4, 2017 March 10, 2017 March 13, 2017 week 1 March 11, 2017 March 17, 2017 March 20, 2017 March 31, 2017 week 2 March 18, 2017 March 24, 2017 March 27, 2017 week 1 March 25, 2017 March 31, 2017 April 3, 2017 April 14, 2017 week 2 April 1, 2017 April 7, 2017 April 10, 2017 week 1 April 8, 2017 April 14, 2017 April 17, 2017 April 28, 2017 week 2 April 15, 2017 April 21, 2017 April 24, 2017 week 1 April 22, 2017 April 28, 2017 May 1, 2017 May 12, 2017 week 2 April 29, 2017 May 5, 2017 May 8, 2017 week 1 May 6, 2017 May 12, 2017 May 15, 2017 May 26, 2017 week 2 May 13, 2017 May 19, 2017 May -

Julian Date Cheat Sheet for Regular Years
Date Code Cheat Sheet For Regular Years Day of Year Calendar Date 1 January 1 2 January 2 3 January 3 4 January 4 5 January 5 6 January 6 7 January 7 8 January 8 9 January 9 10 January 10 11 January 11 12 January 12 13 January 13 14 January 14 15 January 15 16 January 16 17 January 17 18 January 18 19 January 19 20 January 20 21 January 21 22 January 22 23 January 23 24 January 24 25 January 25 26 January 26 27 January 27 28 January 28 29 January 29 30 January 30 31 January 31 32 February 1 33 February 2 34 February 3 35 February 4 36 February 5 37 February 6 38 February 7 39 February 8 40 February 9 41 February 10 42 February 11 43 February 12 44 February 13 45 February 14 46 February 15 47 February 16 48 February 17 49 February 18 50 February 19 51 February 20 52 February 21 53 February 22 54 February 23 55 February 24 56 February 25 57 February 26 58 February 27 59 February 28 60 March 1 61 March 2 62 March 3 63 March 4 64 March 5 65 March 6 66 March 7 67 March 8 68 March 9 69 March 10 70 March 11 71 March 12 72 March 13 73 March 14 74 March 15 75 March 16 76 March 17 77 March 18 78 March 19 79 March 20 80 March 21 81 March 22 82 March 23 83 March 24 84 March 25 85 March 26 86 March 27 87 March 28 88 March 29 89 March 30 90 March 31 91 April 1 92 April 2 93 April 3 94 April 4 95 April 5 96 April 6 97 April 7 98 April 8 99 April 9 100 April 10 101 April 11 102 April 12 103 April 13 104 April 14 105 April 15 106 April 16 107 April 17 108 April 18 109 April 19 110 April 20 111 April 21 112 April 22 113 April 23 114 April 24 115 April -
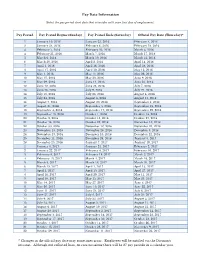
Pay Date Calendar
Pay Date Information Select the pay period start date that coincides with your first day of employment. Pay Period Pay Period Begins (Sunday) Pay Period Ends (Saturday) Official Pay Date (Thursday)* 1 January 10, 2016 January 23, 2016 February 4, 2016 2 January 24, 2016 February 6, 2016 February 18, 2016 3 February 7, 2016 February 20, 2016 March 3, 2016 4 February 21, 2016 March 5, 2016 March 17, 2016 5 March 6, 2016 March 19, 2016 March 31, 2016 6 March 20, 2016 April 2, 2016 April 14, 2016 7 April 3, 2016 April 16, 2016 April 28, 2016 8 April 17, 2016 April 30, 2016 May 12, 2016 9 May 1, 2016 May 14, 2016 May 26, 2016 10 May 15, 2016 May 28, 2016 June 9, 2016 11 May 29, 2016 June 11, 2016 June 23, 2016 12 June 12, 2016 June 25, 2016 July 7, 2016 13 June 26, 2016 July 9, 2016 July 21, 2016 14 July 10, 2016 July 23, 2016 August 4, 2016 15 July 24, 2016 August 6, 2016 August 18, 2016 16 August 7, 2016 August 20, 2016 September 1, 2016 17 August 21, 2016 September 3, 2016 September 15, 2016 18 September 4, 2016 September 17, 2016 September 29, 2016 19 September 18, 2016 October 1, 2016 October 13, 2016 20 October 2, 2016 October 15, 2016 October 27, 2016 21 October 16, 2016 October 29, 2016 November 10, 2016 22 October 30, 2016 November 12, 2016 November 24, 2016 23 November 13, 2016 November 26, 2016 December 8, 2016 24 November 27, 2016 December 10, 2016 December 22, 2016 25 December 11, 2016 December 24, 2016 January 5, 2017 26 December 25, 2016 January 7, 2017 January 19, 2017 1 January 8, 2017 January 21, 2017 February 2, 2017 2 January -

Due Date Chart 201803281304173331.Xlsx
Special Event Permit Application Due Date Chart for Events from January 1, 2019 - June 30, 2020 If due date lands on a Saturday or Sunday, the due date is moved to the next business day Event Date 30 Calendar days 90 Calendar Days Tuesday, January 01, 2019 Sunday, December 02, 2018 Wednesday, October 03, 2018 Wednesday, January 02, 2019 Monday, December 03, 2018 Thursday, October 04, 2018 Thursday, January 03, 2019 Tuesday, December 04, 2018 Friday, October 05, 2018 Friday, January 04, 2019 Wednesday, December 05, 2018 Saturday, October 06, 2018 Saturday, January 05, 2019 Thursday, December 06, 2018 Sunday, October 07, 2018 Sunday, January 06, 2019 Friday, December 07, 2018 Monday, October 08, 2018 Monday, January 07, 2019 Saturday, December 08, 2018 Tuesday, October 09, 2018 Tuesday, January 08, 2019 Sunday, December 09, 2018 Wednesday, October 10, 2018 Wednesday, January 09, 2019 Monday, December 10, 2018 Thursday, October 11, 2018 Thursday, January 10, 2019 Tuesday, December 11, 2018 Friday, October 12, 2018 Friday, January 11, 2019 Wednesday, December 12, 2018 Saturday, October 13, 2018 Saturday, January 12, 2019 Thursday, December 13, 2018 Sunday, October 14, 2018 Sunday, January 13, 2019 Friday, December 14, 2018 Monday, October 15, 2018 Monday, January 14, 2019 Saturday, December 15, 2018 Tuesday, October 16, 2018 2019 Tuesday, January 15, 2019 Sunday, December 16, 2018 Wednesday, October 17, 2018 Wednesday, January 16, 2019 Monday, December 17, 2018 Thursday, October 18, 2018 Thursday, January 17, 2019 Tuesday, December 18, 2018 -

Federal Register/Vol. 85, No. 228/Wednesday, November 25
75720 Federal Register / Vol. 85, No. 228 / Wednesday, November 25, 2020 / Notices DEPARTMENT OF HEALTH AND Support Economic Recovery, 85 FR business information that is included in HUMAN SERVICES 31353 (May 19, 2020) calls on agencies a comment. We post all comments to address the economic emergency received before the close of the Regulatory Relief To Support caused by the COVID–19 pandemic by comment period on the following Economic Recovery; Request for rescinding, modifying, waiving, or website as soon as possible after they Information (RFI) providing exemptions from regulations have been received: https:// and other requirements that may inhibit www.regulations.gov. Follow the search AGENCY: Office of the Secretary, economic recovery, consistent with instructions on that website to view Department of Health and Human applicable law and with protection of public comments. Services. the public health and safety, with ACTION: Request for information. national and homeland security, and I. Background with budgetary priorities and In December 2019, a novel SUMMARY: Under an Executive Order operational feasibility. To implement coronavirus known as SARS–CoV–2 that directs federal agencies to address the directives of E.O. 13924, the U.S. (‘‘the virus’’) was first noted by the the economic emergency created by the Department of Health and Human People’s Republic of China as having COVID–19 pandemic by rescinding, Services (‘‘HHS’’ or ‘‘the Department’’) been detected in Wuhan, Hubei modifying, waiving, or providing identified in in response to this E.O. 382 Province, People’s Republic of China, exemptions from regulations and other regulatory actions that it is considering causing an outbreak of the disease requirements that may inhibit economic to make permanent or keep as COVID–19, which has now spread recovery, consistent with applicable law temporary made in response to the globally. -

Meeting Deadlines
2021 PLANNING COMMISSION DEADLINE DATES Meeting Date Submit Pre-ap Plan Pre-ap Meeting Deadline Application Deadline 10 am Meeting Date January 6, 2021 November 23, 2021 November 25, 2021 December 3, 2020 January 6, 2021 January 20, 2021 December 8, 2020 December 10, 2020 December 17, 2020 January 20, 2021 February 3, 2021 December 14, 2020 December 16, 2020 December 23, 2020 February 3, 2021 February 17, 2021 January 5, 2021 January 7, 2021 January 14, 2021 February 17, 2021 March 3, 2021 January 19, 2021 January 21, 2021 January 28, 2021 March 3, 2021 March 17, 2021 February 2, 2021 February 4, 2021 February 11, 2021 March 17, 2021 April 7, 2021 February 23, 2021 February 25, 2021 March 4, 2021 April 7, 2021 April 21, 2021 March 9, 2021 March 11, 2021 March 18, 2021 April 21, 2021 May 5, 2021 March 23, 2021 March 25, 2021 April 1, 2021 May 5, 2021 May 19, 2021 April 6, 2021 April 8, 2021 April 15, 2021 May 19, 2021 June 2, 2021 April 20, 2021 April 22, 2021 April 29, 2021 June 2, 2021 June 16, 2021 May 4, 2021 May 6, 2021 May 13, 2021 June 16, 2021 July 7, 2021 May 25, 2021 May 27, 2021 June 3, 2021 July 7, 2021 July 21, 2021 June 8, 2021 June 10, 2021 June 17, 2021 July 21, 2021 August 4, 2021 June 22, 2021 June 24, 2021 July 1, 2021 August 4, 2021 August 18, 2021 July 6, 2021 July 8, 2021 July 15, 2021 August 18, 2021 September 1, 2021 July 20, 2021 July 22, 2021 July 29, 2021 September 1, 2021 September 15, 2021 August 3, 2021 August 5, 2021 August 12, 2021 September 15, 2021 October 6, 2021 August 24, 2021 August 26, 2021 September -

Date of Close Contact Exposure
Date of Close Contact Exposure 7 days 10 days 14 days Monday, November 16, 2020 Tuesday, November 24, 2020 Friday, November 27, 2020 Tuesday, December 1, 2020 Tuesday, November 17, 2020 Wednesday, November 25, 2020 Saturday, November 28, 2020 Wednesday, December 2, 2020 Wednesday, November 18, 2020 Thursday, November 26, 2020 Sunday, November 29, 2020 Thursday, December 3, 2020 Thursday, November 19, 2020 Friday, November 27, 2020 Monday, November 30, 2020 Friday, December 4, 2020 Friday, November 20, 2020 Saturday, November 28, 2020 Tuesday, December 1, 2020 Saturday, December 5, 2020 Saturday, November 21, 2020 Sunday, November 29, 2020 Wednesday, December 2, 2020 Sunday, December 6, 2020 Sunday, November 22, 2020 Monday, November 30, 2020 Thursday, December 3, 2020 Monday, December 7, 2020 Monday, November 23, 2020 Tuesday, December 1, 2020 Friday, December 4, 2020 Tuesday, December 8, 2020 Tuesday, November 24, 2020 Wednesday, December 2, 2020 Saturday, December 5, 2020 Wednesday, December 9, 2020 Wednesday, November 25, 2020 Thursday, December 3, 2020 Sunday, December 6, 2020 Thursday, December 10, 2020 Thursday, November 26, 2020 Friday, December 4, 2020 Monday, December 7, 2020 Friday, December 11, 2020 Friday, November 27, 2020 Saturday, December 5, 2020 Tuesday, December 8, 2020 Saturday, December 12, 2020 Saturday, November 28, 2020 Sunday, December 6, 2020 Wednesday, December 9, 2020 Sunday, December 13, 2020 Sunday, November 29, 2020 Monday, December 7, 2020 Thursday, December 10, 2020 Monday, December 14, 2020 Monday, November -

November 25, 1956 the Granma Sets Sail from Mexico
This Month in Latin American History The Granma, as it would have looked in 1956 November 25, 1956 The Granma Sets Sail from Mexico In 1955, having won an uncontested election to validate his reign as president of Cuba, Fulgencio Batista issued a pardon for the nation’s political prisoners, which included both Fidel and Raul Castro, who had been imprisoned for their failed attempt to spark a revolution against Batista on July 26, 1953. Taking advantage of this opportunity, the brothers fled to Mexico, where they began to re-form their organization, which they now dubbed the 26th of July movement. Working with other Cuban exiles (and the Argentine doctor Ernesto “Che” Guevara) and with rebels in the city of Santiago de Cuba, Castro made plans to lead a squad of exiles back to Cuba, with his arrival timed to coincide with an uprising in the city. In October of 1956 he bought the yacht Granma, wanting to use an ordinary civilian craft that would not arouse suspicion. However, the Granma was not fit for the voyage, nor was it built to accommodate the 82 men who set out for Cuba in late November. Castro and his forces arrived late, and the Cuban army was prepared for their arrival- only about 20 men made it off the beach outside of Santiago, fleeing into the mountains in hopes of keeping their movement alive. Today, Granma is the name of the official newspaper of the Cuban Communist Party. Want to know more? Take Latin American History! Contact Prof. Greg Hammond at [email protected] . -

Bulletin Publication Schedule for Calendar Year 2020
BULLETIN PUBLICATION SCHEDULE FOR CALENDAR YEAR 2020 Vol. Monthly Issue ARRF Due Closing Date for 21-day Comment Publication Date No. of Bulletin to DFM Agency Filing Period End Date 20-1 January 2020 November 15, 2019 *November 29, 2019 January 1, 2020 January 22, 2020 20-2 February 2020 December 20, 2019 January 3, 2020 February 5, 2020 February 26, 2020 20-3 March 2020 January 22, 2020 February 7, 2020 March 4, 2020 March 25, 2020 20-4 April 2020 February 21, 2020 March 6, 2020 April 1, 2020 April 22, 2020 20-5 May 2020 March 20, 2020 April 3, 2020 May 6, 2020 May 27, 2020 20-6 June 2020 April 24, 2020 May 1, 2020 June 3, 2020 June 24, 2020 20-7 July 2020 May 22, 2020 June 5, 2020 July 1, 2020 July 22, 2020 20-8 August 2020 June 19, 2020 July 3, 2020 August 5, 2020 August 26, 2020 20-9 September 2020 July 24, 2020 August 7, 2020 September 2, 2020 September 23, 2020 20-10 October 2020 August 21, 2020 **August 28, 2020 October 7, 2020 October 28, 2020 20-11 November 2020 September 18, 2020 October 2, 2020 November 4, 2020 November 25, 2020 20-12 December 2020 October 23, 2020 November 6, 2020 December 2, 2020 December 23, 2020 BULLETIN PUBLICATION SCHEDULE FOR CALENDAR YEAR 2021 Vol. Monthly Issue ARRF Due Closing Date for Bulletin 21-day Comment No. of Bulletin to DFM Agency Filing Publication Date Period End Date 21-1 January 2021 November 16, 2021 *November 30, 2020 January 6, 2021 January 27, 2021 21-2 February 2021 December 24, 2021 January 8, 2021 February 3, 2021 February 24, 2021 21-3 March 2021 January 22, 2021 February 5, 2021