Conservation Concerns for the Candy Darter (Etheostoma Osburni) with Implications Related to Hybridization
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Endangered Species Act 2018
▪ Requires regulators to consider potential effects on T&E species during permitting process ▪ Must evaluate whether they are present ▪ If present, will there be any effects? ▪ Each plant or animal type has particular set of rules about when protective measures need to be placed in permit ▪ Terrestrial species typically only require protections when present within footprint of activity or within a buffer zone of habitat features (roost trees, hibernacula, etc.) ▪ Aquatic species require protections if project is within a certain distance upstream and/or if the project disturbs an upstream drainage area greater than a given size Species Scientific Name Eastern cougar Felis concolor cougar* Indiana bat Myotis sodalis Virginia big-eared bat Corynorhinus townsendii virginianus Northern long-eared bat Myotis septentrionalis Cheat Mountain salamander Plethodon nettingi Diamond darter Crystallaria cincotta Madison Cave isopod Antrolana lira Species Scientific Name Clubshell mussel Pleurobema clava Fanshell mussel Cyprogenia stegaria James spiny mussel Pleurobema collina Pink mucket mussel Lampsilis abrupta Rayed bean mussel Villosa fabalis Sheepnose mussel Plethobasus cyphyus Spectaclecase mussel Cumberlandia monodonta Species Scientific Name Snuffbox mussel Epioblasma triquetra Tubercled blossom pearly mussel Epioblasma torulosa torulosa Guyandotte River crayfish Cambarus veteranus Big Sandy crayfish Canbarus callainus Flat-spired three toothed land snail Triodopsis platysayoides Harperella Ptilimnium nodosum Northeastern bulrush Scirpus ancistrochaetus -
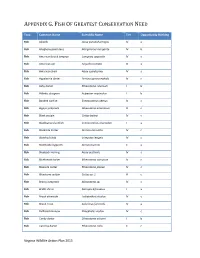
Fish of Greatest Conservation Need
APPENDIX G. FISH OF GREATEST CONSERVATION NEED Taxa Common Name Scientific Name Tier Opportunity Ranking Fish Alewife Alosa pseudoharengus IV a Fish Allegheny pearl dace Margariscus margarita IV b Fish American brook lamprey Lampetra appendix IV c Fish American eel Anguilla rostrata III a Fish American shad Alosa sapidissima IV a Fish Appalachia darter Percina gymnocephala IV c Fish Ashy darter Etheostoma cinereum I b Fish Atlantic sturgeon Acipenser oxyrinchus I b Fish Banded sunfish Enneacanthus obesus IV c Fish Bigeye jumprock Moxostoma ariommum III c Fish Black sculpin Cottus baileyi IV c Fish Blackbanded sunfish Enneacanthus chaetodon I a Fish Blackside darter Percina maculata IV c Fish Blotched chub Erimystax insignis IV c Fish Blotchside logperch Percina burtoni II a Fish Blueback Herring Alosa aestivalis IV a Fish Bluebreast darter Etheostoma camurum IV c Fish Blueside darter Etheostoma jessiae IV c Fish Bluestone sculpin Cottus sp. 1 III c Fish Brassy Jumprock Moxostoma sp. IV c Fish Bridle shiner Notropis bifrenatus I a Fish Brook silverside Labidesthes sicculus IV c Fish Brook Trout Salvelinus fontinalis IV a Fish Bullhead minnow Pimephales vigilax IV c Fish Candy darter Etheostoma osburni I b Fish Carolina darter Etheostoma collis II c Virginia Wildlife Action Plan 2015 APPENDIX G. FISH OF GREATEST CONSERVATION NEED Fish Carolina fantail darter Etheostoma brevispinum IV c Fish Channel darter Percina copelandi III c Fish Clinch dace Chrosomus sp. cf. saylori I a Fish Clinch sculpin Cottus sp. 4 III c Fish Dusky darter Percina sciera IV c Fish Duskytail darter Etheostoma percnurum I a Fish Emerald shiner Notropis atherinoides IV c Fish Fatlips minnow Phenacobius crassilabrum II c Fish Freshwater drum Aplodinotus grunniens III c Fish Golden Darter Etheostoma denoncourti II b Fish Greenfin darter Etheostoma chlorobranchium I b Fish Highback chub Hybopsis hypsinotus IV c Fish Highfin Shiner Notropis altipinnis IV c Fish Holston sculpin Cottus sp. -

Endangered Species
FEATURE: ENDANGERED SPECIES Conservation Status of Imperiled North American Freshwater and Diadromous Fishes ABSTRACT: This is the third compilation of imperiled (i.e., endangered, threatened, vulnerable) plus extinct freshwater and diadromous fishes of North America prepared by the American Fisheries Society’s Endangered Species Committee. Since the last revision in 1989, imperilment of inland fishes has increased substantially. This list includes 700 extant taxa representing 133 genera and 36 families, a 92% increase over the 364 listed in 1989. The increase reflects the addition of distinct populations, previously non-imperiled fishes, and recently described or discovered taxa. Approximately 39% of described fish species of the continent are imperiled. There are 230 vulnerable, 190 threatened, and 280 endangered extant taxa, and 61 taxa presumed extinct or extirpated from nature. Of those that were imperiled in 1989, most (89%) are the same or worse in conservation status; only 6% have improved in status, and 5% were delisted for various reasons. Habitat degradation and nonindigenous species are the main threats to at-risk fishes, many of which are restricted to small ranges. Documenting the diversity and status of rare fishes is a critical step in identifying and implementing appropriate actions necessary for their protection and management. Howard L. Jelks, Frank McCormick, Stephen J. Walsh, Joseph S. Nelson, Noel M. Burkhead, Steven P. Platania, Salvador Contreras-Balderas, Brady A. Porter, Edmundo Díaz-Pardo, Claude B. Renaud, Dean A. Hendrickson, Juan Jacobo Schmitter-Soto, John Lyons, Eric B. Taylor, and Nicholas E. Mandrak, Melvin L. Warren, Jr. Jelks, Walsh, and Burkhead are research McCormick is a biologist with the biologists with the U.S. -

Aquatic Fish Report
Aquatic Fish Report Acipenser fulvescens Lake St urgeon Class: Actinopterygii Order: Acipenseriformes Family: Acipenseridae Priority Score: 27 out of 100 Population Trend: Unknown Gobal Rank: G3G4 — Vulnerable (uncertain rank) State Rank: S2 — Imperiled in Arkansas Distribution Occurrence Records Ecoregions where the species occurs: Ozark Highlands Boston Mountains Ouachita Mountains Arkansas Valley South Central Plains Mississippi Alluvial Plain Mississippi Valley Loess Plains Acipenser fulvescens Lake Sturgeon 362 Aquatic Fish Report Ecobasins Mississippi River Alluvial Plain - Arkansas River Mississippi River Alluvial Plain - St. Francis River Mississippi River Alluvial Plain - White River Mississippi River Alluvial Plain (Lake Chicot) - Mississippi River Habitats Weight Natural Littoral: - Large Suitable Natural Pool: - Medium - Large Optimal Natural Shoal: - Medium - Large Obligate Problems Faced Threat: Biological alteration Source: Commercial harvest Threat: Biological alteration Source: Exotic species Threat: Biological alteration Source: Incidental take Threat: Habitat destruction Source: Channel alteration Threat: Hydrological alteration Source: Dam Data Gaps/Research Needs Continue to track incidental catches. Conservation Actions Importance Category Restore fish passage in dammed rivers. High Habitat Restoration/Improvement Restrict commercial harvest (Mississippi River High Population Management closed to harvest). Monitoring Strategies Monitor population distribution and abundance in large river faunal surveys in cooperation -

A List of Common and Scientific Names of Fishes from the United States And
t a AMERICAN FISHERIES SOCIETY QL 614 .A43 V.2 .A 4-3 AMERICAN FISHERIES SOCIETY Special Publication No. 2 A List of Common and Scientific Names of Fishes -^ ru from the United States m CD and Canada (SECOND EDITION) A/^Ssrf>* '-^\ —---^ Report of the Committee on Names of Fishes, Presented at the Ei^ty-ninth Annual Meeting, Clearwater, Florida, September 16-18, 1959 Reeve M. Bailey, Chairman Ernest A. Lachner, C. C. Lindsey, C. Richard Robins Phil M. Roedel, W. B. Scott, Loren P. Woods Ann Arbor, Michigan • 1960 Copies of this publication may be purchased for $1.00 each (paper cover) or $2.00 (cloth cover). Orders, accompanied by remittance payable to the American Fisheries Society, should be addressed to E. A. Seaman, Secretary-Treasurer, American Fisheries Society, Box 483, McLean, Virginia. Copyright 1960 American Fisheries Society Printed by Waverly Press, Inc. Baltimore, Maryland lutroduction This second list of the names of fishes of The shore fishes from Greenland, eastern the United States and Canada is not sim- Canada and the United States, and the ply a reprinting with corrections, but con- northern Gulf of Mexico to the mouth of stitutes a major revision and enlargement. the Rio Grande are included, but those The earlier list, published in 1948 as Special from Iceland, Bermuda, the Bahamas, Cuba Publication No. 1 of the American Fisheries and the other West Indian islands, and Society, has been widely used and has Mexico are excluded unless they occur also contributed substantially toward its goal of in the region covered. In the Pacific, the achieving uniformity and avoiding confusion area treated includes that part of the conti- in nomenclature. -

Candy Darter Recovery Outline
U.S. Fish & Wildlife Service Candy Darter Recovery Outline Photo by Corey Dunn, University of Missouri Species Name: Candy Darter (Etheostoma osburni) Species Range: Upper Kanawha River Basin including the Gauley, Greenbrier, and New River Watersheds including portions of Greenbrier, Pocahontas, Nicholas, and Webster counties in West Virginia; and Bland, Giles, and Wythe counties, Virginia. The range of the species is shown in figure 1 below. Recovery Priority Number: 5; explanation provided below Listing Status: Endangered; November 21, 2019; 83 FR 58747 Lead Regional Office/Cooperating RO(s): Northeast Region, Hadley MA Lead Field Office/Cooperating FO(s): West Virginia Field Office, Elkins WV; Southwestern Virginia Field Office, Abingdon, VA; White Sulphur Springs National Fish Hatchery, White Sulphur Springs, WV Lead Contact: Barbara Douglas, 304-636-6586 ext 19. [email protected] 1) Background This section provides a brief overview of the ecology and conservation of the candy darter. This information is more fully described in the Species Status Assessment (SSA), the final listing rule, and the proposed critical habitat rule. These documents are available at: https://www.fws.gov/northeast/candydarter/ 1 Type and Quality of Available Information to Date: Important Information Gaps and Treatment of Uncertainties One of the primary threats that resulted in the listing of the candy darter under the Endangered Species Act (ESA) is the spread of the introduced variegate darter (Etheostoma variatum). This species hybridizes with the candy darter. Key information gaps and areas of uncertainty for the recovery and management of the candy darter include whether there are any habitat or other natural factors that could limit the spread of variegate darter. -

Checklist of Arkansas Fishes Thomas M
Journal of the Arkansas Academy of Science Volume 27 Article 11 1973 Checklist of Arkansas Fishes Thomas M. Buchanan University of Arkansas – Fort Smith Follow this and additional works at: http://scholarworks.uark.edu/jaas Part of the Population Biology Commons, and the Terrestrial and Aquatic Ecology Commons Recommended Citation Buchanan, Thomas M. (1973) "Checklist of Arkansas Fishes," Journal of the Arkansas Academy of Science: Vol. 27 , Article 11. Available at: http://scholarworks.uark.edu/jaas/vol27/iss1/11 This article is available for use under the Creative Commons license: Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0). Users are able to read, download, copy, print, distribute, search, link to the full texts of these articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author. This Article is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Journal of the Arkansas Academy of Science by an authorized editor of ScholarWorks@UARK. For more information, please contact [email protected], [email protected]. Journal of the Arkansas Academy of Science, Vol. 27 [1973], Art. 11 Checklist of Arkansas Fishes THOMAS M.BUCHANAN Department ot Natural Science, Westark Community College, Fort Smith, Arkansas 72901 ABSTRACT Arkansas has a large, diverse fish fauna consisting of 193 species known to have been collected from the state's waters. The checklist is an up-to-date listing of both native and introduced species, and is intended to correct some of the longstanding and more recent erroneous Arkansas records. -

Proceedings of the United States National Museum
Proceedings of the United States National Museum SMITHSONIAN INSTITUTION . WASHINGTON, D.C. Volume 119 1966 Number 3550 CATALOG OF TYPE SPECIMENS OF THE DARTERS (PISCES, PERCIDAE, ETHEOSTOMATINI) ^ By Bruce B. Collette and Leslie W. Knapp ^ Introduction The darters are a tribe of small freshwater fishes restricted to North America. Some 212 specific and subspecific names have been pro- posed for the approximately 100 vahd described species. About a dozen species await description. As part of a long-term study of these fishes, we have prepared this catalog of type specimens. We hope that our efforts will be of value in furthering systematic research and stabilizing the nomenclature of this most fascinating group of North American fishes. In preparing this catalog we have attempted to examine or at least to verify the location of the type specimens of all nominal forms of the three presently recognized genera of darters: Percina, Ammo- crypta, and Etheostoma. By type specimens we mean holotypes, lectotypes, syntypes, paralectotypes, and paratypes. Each form is ' Fifth paper in a series on the systematics of the Percidae by the senior author. 2 Collette: Assistant Laboratory Director, Bureau of Commercial Fisheries Ichthyological Laboratory, Division of Fishes, U.S. National Museum; Knapp: Supervisor in charge of vertebrates, Oceanographic Sorting Center, Smithsonian Institution. a 2 PROCEEDINGS OF THE NATIONAL MUSEUM vol. 119 listed in alphabetical order by the generic and specific name used in the original description. If subgeneric allocation was made in the original description, that name has been placed in parentheses between the genus and the species. Holotypes or lectotypes are listed before paratypes or paralectotypes. -

Arkansas Endemic Biota: an Update with Additions and Deletions H
Journal of the Arkansas Academy of Science Volume 62 Article 14 2008 Arkansas Endemic Biota: An Update with Additions and Deletions H. Robison Southern Arkansas University, [email protected] C. McAllister C. Carlton Louisiana State University G. Tucker FTN Associates, Ltd. Follow this and additional works at: http://scholarworks.uark.edu/jaas Part of the Botany Commons Recommended Citation Robison, H.; McAllister, C.; Carlton, C.; and Tucker, G. (2008) "Arkansas Endemic Biota: An Update with Additions and Deletions," Journal of the Arkansas Academy of Science: Vol. 62 , Article 14. Available at: http://scholarworks.uark.edu/jaas/vol62/iss1/14 This article is available for use under the Creative Commons license: Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0). Users are able to read, download, copy, print, distribute, search, link to the full texts of these articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author. This Article is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Journal of the Arkansas Academy of Science by an authorized editor of ScholarWorks@UARK. For more information, please contact [email protected], [email protected]. Journal of the Arkansas Academy of Science, Vol. 62 [2008], Art. 14 The Arkansas Endemic Biota: An Update with Additions and Deletions H. Robison1, C. McAllister2, C. Carlton3, and G. Tucker4 1Department of Biological Sciences, Southern Arkansas University, Magnolia, AR 71754-9354 2RapidWrite, 102 Brown Street, Hot Springs National Park, AR 71913 3Department of Entomology, Louisiana State University, Baton Rouge, LA 70803-1710 4FTN Associates, Ltd., 3 Innwood Circle, Suite 220, Little Rock, AR 72211 1Correspondence: [email protected] Abstract Pringle and Witsell (2005) described this new species of rose-gentian from Saline County glades. -

Status Survey and Population Characteristics of the Paleback Darter (Etheostoma Pallididorsum) and the Caddo Madtom (Noturus Taylori)
Title: Status survey and population characteristics of the Paleback Darter (Etheostoma pallididorsum) and the Caddo Madtom (Noturus taylori) Project Summary: This project seeks to provide updated information on the status and population characteristics of two of Arkansas’ Species of Greatest Conservation Need, the Paleback Darter and Caddo Madtom. The main objectives of this project include: (1) conducting surveys of historic localities throughout their range; (2) obtaining size-distribution (estimating age classes) data and relative abundance for both species at select locations on a seasonal basis; and (3) generating population characteristics for each species via non-lethal genetic methods (e.g. genetic variation, effective population sizes, patterns of migration). The overall objective is to provide data for both species that will inform potential listing decisions under the Endangered Species Act by the U.S. Fish and Wildlife Service, in addition to state ranking decisions by the Arkansas Game and Fish Commission. Principle Investigator: Dr. Brook L. Fluker (Assistant Professor of Aquatic Ecology) Arkansas State University, Department of Biological Sciences Po Box 599, State University, AR 72467 Phone: (870)-972-3253 email: [email protected] Project Partners: 1. Betty Crump (Stream Ecologist) US Forest Service, Ouachita National Forest, 100 Reserve Street, Hot Springs, AR 71901 Phone: 501-321-5236 email: [email protected] 2. Mitzi Cole (Fishery Biologist) US Forest Service, Ouachita National Forest, 100 Reserve Street, Hot Springs, -
![Kyfishid[1].Pdf](https://docslib.b-cdn.net/cover/2624/kyfishid-1-pdf-1462624.webp)
Kyfishid[1].Pdf
Kentucky Fishes Kentucky Department of Fish and Wildlife Resources Kentucky Fish & Wildlife’s Mission To conserve, protect and enhance Kentucky’s fish and wildlife resources and provide outstanding opportunities for hunting, fishing, trapping, boating, shooting sports, wildlife viewing, and related activities. Federal Aid Project funded by your purchase of fishing equipment and motor boat fuels Kentucky Department of Fish & Wildlife Resources #1 Sportsman’s Lane, Frankfort, KY 40601 1-800-858-1549 • fw.ky.gov Kentucky Fish & Wildlife’s Mission Kentucky Fishes by Matthew R. Thomas Fisheries Program Coordinator 2011 (Third edition, 2021) Kentucky Department of Fish & Wildlife Resources Division of Fisheries Cover paintings by Rick Hill • Publication design by Adrienne Yancy Preface entucky is home to a total of 245 native fish species with an additional 24 that have been introduced either intentionally (i.e., for sport) or accidentally. Within Kthe United States, Kentucky’s native freshwater fish diversity is exceeded only by Alabama and Tennessee. This high diversity of native fishes corresponds to an abun- dance of water bodies and wide variety of aquatic habitats across the state – from swift upland streams to large sluggish rivers, oxbow lakes, and wetlands. Approximately 25 species are most frequently caught by anglers either for sport or food. Many of these species occur in streams and rivers statewide, while several are routinely stocked in public and private water bodies across the state, especially ponds and reservoirs. The largest proportion of Kentucky’s fish fauna (80%) includes darters, minnows, suckers, madtoms, smaller sunfishes, and other groups (e.g., lam- preys) that are rarely seen by most people. -

And the Eastern Sand Darter (Ammocrypta Pellucida) in the Elk River, West Virginia
Graduate Theses, Dissertations, and Problem Reports 2016 Distribution and habitat use of the western sand darter (Ammocrypta clara) and the eastern sand darter (Ammocrypta pellucida) in the Elk River, West Virginia Patricia A. Thompson Follow this and additional works at: https://researchrepository.wvu.edu/etd Recommended Citation Thompson, Patricia A., "Distribution and habitat use of the western sand darter (Ammocrypta clara) and the eastern sand darter (Ammocrypta pellucida) in the Elk River, West Virginia" (2016). Graduate Theses, Dissertations, and Problem Reports. 6800. https://researchrepository.wvu.edu/etd/6800 This Thesis is protected by copyright and/or related rights. It has been brought to you by the The Research Repository @ WVU with permission from the rights-holder(s). You are free to use this Thesis in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you must obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This Thesis has been accepted for inclusion in WVU Graduate Theses, Dissertations, and Problem Reports collection by an authorized administrator of The Research Repository @ WVU. For more information, please contact [email protected]. Distribution and habitat use of the western sand darter (Ammocrypta clara) and the eastern sand darter (Ammocrypta pellucida) in the Elk River, West Virginia Patricia A. Thompson Thesis submitted to the Davis College of Agriculture, Natural Resources and Design at West Virginia University in partial fulfillment of the requirements for the degree of Master of Science in Wildlife and Fisheries Resources Stuart A.