Importance of Neem Tree
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Multicomponent Distillation
01/12/2009 Multicomponent distillation F. Grisafi 1 01/12/2009 Introduction The problem of determining the stage and reflux requitirements for multicomponen t distill a tions ismuch more complex than for binary mixtures. With a multicomponent mixture, fixing one component composition does not uniquely determine theother component compositions and the stage temperature. Also when the feed contains more than two components it is not possible to specify the complete composition of the top and bottom products independently. The separation between the top and bottom products is usually specified by setting limits on two "key components", between which it is desired to make the separation. 2 01/12/2009 Calculation procedure The normal procedure for a typical problem is to solve the MESH (Material balance, Equilibrium, Summation and Heat) balance equations stage-by-stage, from the top and bottom of the column toward the feed point. For such a calculation to be exact, the compositions obtained from both the bottom-up and top-down calculations must mesh at the feed point and mesh the feed composition. The calculated compositions will depend on the compositions assumed for the top and bottom products at the commencement of the calculations. Though it ispossibletomatch the key components, theother components will not match unless the designer was particularly fortunate in choosing the trial top and bottom compositions. 3 01/12/2009 Calculation procedure For a comppyletely rigorous solution the compositions must be adjusted and the calculations repeated until a satisfactory match at the feed point is obtained by iterative trial-and-error calculations. Clearly, the greater the number of components, the more difficult the problem. -

NEEM: the Divine Tree, Azadirachta Indica
NEEM Copyright © 1999 OPA (Overseas Publishers Association) N.V. Published by license under the Harwood Academic Publishers imprint, part of The Gordon and Breach Publishing Group. Medicinal and Aromatic Plants—Industrial Profiles Individual volumes in this series provide both industry and academia with in-depth coverage of one major medicinal or aromatic plant of industrial importance. Edited by Dr Roland Hardman Volume 1 Valerian edited by Peter J.Houghton Volume 2 Perilla edited by He-Ci Yu, Kenichi Kosuna and Megumi Haga Volume 3 Poppy edited by Jeno Bernáth Volume 4 Cannabis edited by David T.Brown Volume 5 Neem H.S.Puri Other volumes in preparation Allium, edited by K.Chan Artemisia, edited by C.Wright Basil, edited by R.Hiltunen and Y.Holm Caraway, edited by É. Németh Cardamom, edited by PN.Ravindran and KJ.Madusoodanan Chamomile, edited by R.Franke and H.Schilcher Cinnamon and Cassia, edited by P.N.Ravindran and S.Ravindran Colchicum, edited by V.Simánek Curcuma, edited by B.A.Nagasampagi and A.P.Purohit Ergot, edited by V.Kren and L.Cvak Eucalyptus, edited by J.Coppen Ginkgo, edited by T.van Beek Ginseng, by W.Court Hypericum, edited by K.Berger Buter and B.Buter Illicium and Pimpinella, edited by M.Miró Jodral Kava, edited by Y.N.Singh Licorice, by L.E.Craker, L.Kapoor and N.Mamedov Piper Nigrum, edited by P.N.Ravindran Plantago, edited by C.Andary and S.Nishibe Please see the back of this book for other volumes in preparation in Medicinal and Aromatic Plants—Industrial Profiles Copyright © 1999 OPA (Overseas Publishers Association) N.V. -

Example: Multicomponent Distillation with Shortcut Methods Consider A
2 Example: multicomponent distillation with shortcut methods Consider a single distillation column as shown in Fig. 6-1. Short-cut methods have been developed that allow you to see the splits that can be achieved at different pressures, with different reflux ratios, and with a different number of stages. It is often useful to do a short-cut distillation calculation first, before doing the more rigorous plate- to-plate calculations, because the preliminary calculations give you an idea of what is reasonable. The methods are based on the relative volatility of the chemicals, and require you to identify two components that will be essentially separated (to the extent you specify). If you line up all the components in the order of their boiling points, and draw a line between two of them, the more volatile component is called the light key and the less volatile component is called the heavy key. B1 Figure 6-1. Distillation Column For this example, you choose the first column in the process shown in Fig. 5-4. In Aspen Plus you use the module DSTWU for the shortcut method; you also use RK-Soave as the physical property method, because it is a good one for hydrocarbons. The feed is 100 lb. moles/hr of propane, 300 lb. moles/hr of i-butane, and the other chemicals as listed in Table 5-1 or Table 6-6, at 138 psia and 75 ºF. The column operates at 138 psia with a reflux ratio of 10 (a wild1 guess initially, confirmed because the column worked). -

Dimension Growth of Azadirachta Excelsa and Phyllanthus Spp. in Agroforestry System
BIODIVERSITAS ISSN: 1412-033X Volume 18, Number 2, April 2017 E-ISSN: 2085-4722 Pages: 494-499 DOI: 10.13057/biodiv/d180207 Dimension growth of Azadirachta excelsa and Phyllanthus spp. in agroforestry system NILASARI DEWI1,♥, NURHENI WIJAYANTO2,♥♥, GUSMAINI3 1 Program of Tropical Silviculture, School of Graduates, Institut Pertanian Bogor. Jl. Lingkar Akademik Kampus IPB Darmaga, Bogor 16680, West ♥ Java, Indonesia. email: [email protected] 2Department of Silviculture, Faculty of Forestry, Institut Pertanian Bogor. Jl. Lingkar Akademik Kampus IPB Darmaga, Bogor 16680, West Java, ♥♥ Indonesia. Tel. +62-251-8626806, email: [email protected] 3Research Center for Spice and Medical Plant. Bogor 16111, West Java, Indonesia Manuscript received: 2 December 2016. Revision accepted: 15 February 2017. Abstract. Dewi N, Wijayanto N, Gusmaini. 2017. Dimension growth of Azadirachta excelsa and Phyllanthus spp. in agroforestry system. Biodiversitas 18: 494-499. Azadirachta excelsa Jack. is one of the fast growing species which have high resistance to pest and disease, good quality of wood, and high economic value. A. excelsa planting can be integrated with Phyllanthus spp. in agroforestry system. The research about meniran and sentang in agroforestry system was conducted to analyze the influence of A. excelsa allelopathy towards the growth of meniran and to analyze the growth of both plants. This research was conducted for six months in Bogor, West Java. This study was divided into three parts, (i) analyze the effect of allelopathy in A. excelsa leaf and twig on the growth of P. urinaria and Phyllanthus debilis, (ii) analyze the growth of A. excelsa in monoculture and agroforestry systems and (iii) analyze the growth of meniran in monoculture and agroforestry systems. -
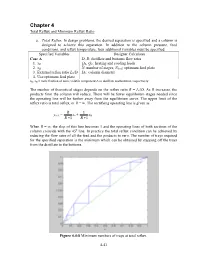
Minimum Reflux Ratio
Chapter 4 Total Reflux and Minimum Reflux Ratio a. Total Reflux . In design problems, the desired separation is specified and a column is designed to achieve this separation. In addition to the column pressure, feed conditions, and reflux temperature, four additional variables must be specified. Specified Variables Designer Calculates Case A D, B: distillate and bottoms flow rates 1. xD QR, QC: heating and cooling loads 2. xB N: number of stages, Nfeed : optimum feed plate 3. External reflux ratio L0/D DC: column diameter 4. Use optimum feed plate xD, xB = mole fraction of more volatile component A in distillate and bottoms, respectively The number of theoretical stages depends on the reflux ratio R = L0/D. As R increases, the products from the column will reduce. There will be fewer equilibrium stages needed since the operating line will be further away from the equilibrium curve. The upper limit of the reflux ratio is total reflux, or R = ∞. The rectifying operating line is given as R 1 yn+1 = xn + xD R +1 R +1 When R = ∞, the slop of this line becomes 1 and the operating lines of both sections of the column coincide with the 45 o line. In practice the total reflux condition can be achieved by reducing the flow rates of all the feed and the products to zero. The number of trays required for the specified separation is the minimum which can be obtained by stepping off the trays from the distillate to the bottoms. Figure 4.4-8 Minimum numbers of trays at total reflux. -

The One Hundred Tree Species Prioritized for Planting in the Tropics and Subtropics As Indicated by Database Mining
The one hundred tree species prioritized for planting in the tropics and subtropics as indicated by database mining Roeland Kindt, Ian K Dawson, Jens-Peter B Lillesø, Alice Muchugi, Fabio Pedercini, James M Roshetko, Meine van Noordwijk, Lars Graudal, Ramni Jamnadass The one hundred tree species prioritized for planting in the tropics and subtropics as indicated by database mining Roeland Kindt, Ian K Dawson, Jens-Peter B Lillesø, Alice Muchugi, Fabio Pedercini, James M Roshetko, Meine van Noordwijk, Lars Graudal, Ramni Jamnadass LIMITED CIRCULATION Correct citation: Kindt R, Dawson IK, Lillesø J-PB, Muchugi A, Pedercini F, Roshetko JM, van Noordwijk M, Graudal L, Jamnadass R. 2021. The one hundred tree species prioritized for planting in the tropics and subtropics as indicated by database mining. Working Paper No. 312. World Agroforestry, Nairobi, Kenya. DOI http://dx.doi.org/10.5716/WP21001.PDF The titles of the Working Paper Series are intended to disseminate provisional results of agroforestry research and practices and to stimulate feedback from the scientific community. Other World Agroforestry publication series include Technical Manuals, Occasional Papers and the Trees for Change Series. Published by World Agroforestry (ICRAF) PO Box 30677, GPO 00100 Nairobi, Kenya Tel: +254(0)20 7224000, via USA +1 650 833 6645 Fax: +254(0)20 7224001, via USA +1 650 833 6646 Email: [email protected] Website: www.worldagroforestry.org © World Agroforestry 2021 Working Paper No. 312 The views expressed in this publication are those of the authors and not necessarily those of World Agroforestry. Articles appearing in this publication series may be quoted or reproduced without charge, provided the source is acknowledged. -

Soft Matter Simulation Notes
Advanced Engineering Separations 2019-2020 Thomas Rodgers UG Notes Contents List of Figures vii List of Tables ix Nomenclature xi Course Information xiii 1 Industrial Separations1 1.1 Chapter1 ILOs...............................2 1.2 Introduction.................................3 1.3 Basic Separation Techniques........................3 1.4 Separations by Phase Addition or Creation.................5 1.5 Separations by Barriers........................... 11 1.6 Separations by Solid Agents........................ 14 1.7 Separations by External Field or Gradient................. 16 1.8 Component Recoveries........................... 19 1.8.1 Split Fractions and Split Ratios.................. 19 1.8.2 Separation Factor.......................... 19 1.9 References.................................. 20 1.10 Problems.................................. 21 2 Liquid-Liquid Extraction 27 2.1 Chapter2 ILOs............................... 28 2.2 Introduction................................. 29 2.2.1 Partition coefficient......................... 30 2.2.2 Solvent Selection.......................... 30 2.3 General Design Considerations....................... 32 2.4 Representation of Multi-phase Liquid-Liquid Systems........... 33 2.5 Single Stage Liquid-Liquid Extraction................... 34 2.6 Lever-Arm Rule............................... 36 2.7 Hunter-Nash Graphical Equilibrium-Stage Method............ 37 2.7.1 Step 1 - Calculation of the Mixing Point.............. 38 2.7.2 Step 2 - Product Mass Balance................... 38 2.7.3 Step 3 - Calculation of the Operating Lines............ 39 2.7.4 Step 4 - Tie Lines and Equilibrium Lines............. 40 2.8 Minimum and Maximum Solvent-to-Feed Flow-Rate Ratios....... 41 2.8.1 Minimum Ratio........................... 42 i 2.8.2 Maximum Ratio.......................... 42 2.9 Equipment for Solvent Extraction..................... 45 2.9.1 Mixer-Settlers............................ 45 2.9.2 Spray Columns........................... 46 2.9.3 Packed Columns.......................... 47 2.9.4 Plate Columns.......................... -

Some Histo-Physiological Effects of Azadirachta Excelsa (Jack) Leaf Extract on Reproductive Organs and Fertility of Female Albino Mice (Mus Musculus)
------ Raf. J. Sci., Vol. 24, No.6 pp. 1-9, 2013------ Some Histo-physiological Effects of Azadirachta excelsa (Jack) Leaf Extract on Reproductive Organs and Fertility of Female albino Mice (Mus musculus) Waad S. Shaher Department of Biology College of Science University of Mosul (Received 30 / 4 / 2013 ; Accepted 10 / 6 / 2013) ABSTRACT This study was conducted to investigate the effect of ethanol leaf extract of Azadirachta excelsa oral dose (250 mg /kg of b.w.) every other day for 28 days on the histological changes of the ovary and uterus, serum levels of progesterone and estrogen and the fertility of adult female mice. The results showed histological changes in the ovaries of the treated groups indicating an increase in the number of secondary follicles, absence of the mature follicles, presence of the atretic follicles, bleeding tissue and congestion of blood vessels in the second group, while in the third group, they showed a decrease in the number of the primary and secondary follicles, absence of the mature follicles, presence of the atretic follicles and fibrosis of the ovarian cortex. The uterus sections of the treated groups showed fibrosis of the uterus wall, hyperplasia of smooth muscle in the second group and papilloma of endometrium, congestion of blood vessels of serosa in the third group. The results observed a significant increase in progesterone and estrogen levels of the treated groups compared with control. The results observed an obvious decrease in the mean of newborn of the treated groups, which proved reduction of the fertility of the treated females. Thus, the application of this plant extract is important in the rodent control programs. -

Distillation Column Selection and Sizing Rev: 03 Practical Engineering Guidelines for Processing Plant (ENGINEERING DESIGN GUIDELINES) Solutions Feb 2011
Page : 1 of 82 KLM Technology Group Rev: 03 Practical Engineering Rev 1 Feb 2007 Guidelines for Processing Plant Rev 2 April 2008 Solutions www.klmtechgroup.com Rev 3 Feb 2011 Author: KLM Technology Group Rev 1 Chew Yin Hoon #03-12 Block Aronia, Rev 2 Ai Li Ling Riverria Condovilla, Distillation Column Rev 3 Aprilia Jaya Taman Tampoi Utama Selection and Sizing Jalan Sri Perkasa 2 Checked by: 81200 Johor Bahru Malaysia (ENGINEERING DESIGN GUIDELINES) Karl Kolmetz TABLE OF CONTENTS INTRODUCTION Scope 6 Distillation 7 Distillation History 7 Types of Distillation Processes 9 Mode of Operation 10 Column Internals 11 Types of Distillation Column 15 A. Tray Column 15 A.1 Tray Hydraulic 18 B. Packed Column 21 B.1 Packed Hydraulic 23 General Design Consideration 26 The Selection of Column Internals 28 DEFINITIONS 34 This design guideline are believed to be as accurate as possible, but are very general not for specific design cases. They were designed for engineers to do preliminary designs and process specification sheets. The final design must always be guaranteed for the service selected by the manufacturing vendor, but these guidelines will greatly reduce the amount of up front engineering hours that are required to develop the final design. The guidelines are a training tool for young engineers or a resource for engineers with experience. This document is entrusted to the recipient personally, but the copyright remains with us. It must not be copied, reproduced or in any way communicated or made accessible to third parties without our written -

In Vitro Propagation of Tropical Hardwood Tree Species – a Review (2001-2011)
Propagation of Ornamental Plants Vol. 12, № 1, 2012: 25-51 Invited paper IN VITRO PROPAGATION OF TROPICAL HARDWOOD TREE SPECIES – A REVIEW (2001-2011) Paula M. Pijut1*, Rochelle R. Beasley2, Shaneka S. Lawson2, Kaitlin J. Palla2, Micah E. Stevens2, and Ying Wang2 1USDA Forest Service, Northern Research Station, Hardwood Tree Improvement and Regeneration Center (HTIRC), 715 West State Street, West Lafayette, Indiana, USA 47907 *Fax: + 1-765-494-9461, *E-mail: [email protected] 2Purdue University, Department of Forestry and Natural Resources, HTIRC, 715 West State Street, West Lafayette, Indiana, USA 47907 Abstract Tropical hardwood tree species are important economically and ecologically, and play a significant role in the biodiversity of plant and animal species within an ecosystem. There are over 600 species of tropical timbers in the world, many of which are commercially valuable in the international trade of plywood, roundwood, sawnwood, and veneer. Many of these tree species are being threatened and are endangered because of logging practices, conversion to agricultural lands, non-optimal management strategies, and overall deforestation rates that cannot keep up with natural regeneration of native forests. Tropical tree species provide timber for com- mercial uses because of the beauty of the wood-grain, -color, or -pattern, strength, durability, and versatility of finishing applications for a vast array of markets. Because of the high value of tropical tree species,in vitro (adventitious shoot regeneration, cryopreservation, genetic transformation, micrografting, protoplast culture, shoot tip and nodal culture, and somatic embryogenesis) propagation technologies are an integral component in tree improvement and conservation programs, in order to complement seed banking and ex situ measures for long-term conservation and clonal propagation of germplasm. -

Effect of Azadirachta Excelsa (Jack) Leaf Extracts on the Reproductive Organs and Fertility of Male Albino Mice (Mus Musculus)
------Jou. Raf. Sci., Vol. 20, No.3 pp 1- 9, 2009- ----- Effect of Azadirachta excelsa (Jack) Leaf Extracts on the Reproductive Organs and Fertility of Male albino Mice (Mus musculus) Waad S. Shaher Department of Biology College of Science Mosul University (Received 3 / 3/ 2009 ; Accepted 15 / 6 / 2009) ABSTRACT This study was conducted to investigate the effect of oral dose (250mg/kg body weight/day, for 21 days) of each aqueous and alcohol leaf extract of Azadirachta excelsa on histological structure of the testis and fertility of male albino mice Mus musculus. Histological structure of the testis of both treated groups showed affected seminiferous tubules indicating mixing of the germ cell types in stages of spermatogenesis, atrophy of the spermatogenic elements, increases in number of Leydig cells, occurrence of giant cells and decrease s or absence of the spermatozoa in the lumen of the seminiferous tubules as compared with control group. The other alternations of both treated groups were decrease in number of the spermatozoa in the Ductus epididymidis. The fertility index of the treated groups was reduced, this result which proves the fertility was observed in untreated females after mated with treated males. Keyword: Azadirachta excelsa, Leaf extract, Testis, Histopathology, Spermatozoa, Fertility. ـــــــــــــــــــــــــــــــــــــــــــــــــــ ﺘﺄﺜﻴﺭ ﻤﺴﺘﺨﻠﺼﺎﺕ ﺍﻭﺭﺍﻕ Azadirachta excelsa ﻋﻠﻰ ﺍﻻﻋﻀﺎﺀ ﺍﻟﺘﻜﺎﺜﺭﻴﺔ ﻭﺨﺼﻭﺒﺔ ﺫﻜﻭﺭ Mus Musculus ﺍﻟﻔﺌﺭﺍﻥ ﺍﻟﺒﻴﺽ ﺍﻟﺴﻭﻴﺴﺭﻴﺔ ﺍﻟﻤﻠﺨﺹ ﺍﺠﺭﻴﺕ ﻫﺫﻩ ﺍﻟﺩﺭﺍﺴﺔ ﻟﻤﻼﺤﻅﺔ ﺘﺄﺜﻴﺭ ﺍﻟﺠﺭﻋﺔ ﺍﻟﻔﻤﻴﺔ (250 ﻤﻠﻐﻡ /ﻜﻐﻡ ﻤﻥ ﻭﺯﻥ ﺍﻟﺠﺴﻡ ﻟﻤﺩﺓ 21 ﻴﻭﻤﺎ ) ﻟﻜل ﻤﻥ ﺍﻟﻤﺴﺘﺨﻠﺼﻴﻥ ﺍﻟﻤﺎﺌﻲ ﻭﺍﻟﻜﺤﻭﻟﻲ ﻻﻭﺭﺍﻕ ﻨﺒﺎﺕ Azadirachta excelsa ﻋﻠﻰ ﺍﻟﺘﺭﻜﻴﺏ ﺍﻟﻨـﺴﺠﻲ ﻟﻠﺨـﺼﻰ ﻭﺍﻟﺨﺼﻭﺒﺔ ﻟﺫﻜﻭﺭ ﺍﻟﻔﺌﺭﺍﻥ ﺍﻟﺒﻴﺽ ﺍﻟﺴﻭﻴﺴﺭﻴﺔ Mus musculus. ﺍﻟﺘﺭﻜﻴﺏ ﺍﻟﻨﺴﺠﻲ ﻓﻲ ﺍﻟﺨﺼﻴﺔ ﻟﻜﻼ ﺍﻟﻤﺠﺎﻤﻴﻊ ﺍﻟﻤﻌﺎﻤﻠﺔ ﺍﻅﻬﺭ ﻨﺒﻴﺒﺎﺕ ﻤﻨﻭﻴﺔ ﻤﺘﺄﺜﺭﺓ ﺘﻀﻤﻨﺕ ﻤﺯﺝ ﻭﻋﺩﻡ ﺍﻨﺘﻅﺎﻡ ﺍﻨﻭﺍﻉ ﺍﻟﺨﻼﻴﺎ ﺍﻟﺠ ﺭﺜﻭﻤﻴﺔ ﻓﻲ ﻤﺭﺍﺤـل ﺘﻜـﻭﻴﻥ ﺍﻟﻨﻁﺎﻑ، ﻭﻀﻤﻭﺭ ﺍﻟﻌﻨﺎﺼﺭ ﺍﻟﻤﻨﻁﻔﺔ، ﻭﺍﺯﺩﻴﺎﺩ ﻓﻲ ﺍﻋﺩﺍﺩ ﺨﻼﻴﺎ ﻟﻴﺩﺝ، ﻭﻅﻬﻭﺭ ﺨﻼﻴﺎ ﻋﻤﻼﻗـﺔ، ﻭﺍﻨﺨﻔـﺎﺽ ﺍﻭ 1 2 Waad S. -

A Short-Cut Method for Predicting the Tot~ Reflux Performance of Distillation
A SHORT-CUT METHOD FOR PREDICTING THE TOT~ REFLUX PERFORMANCE OF DISTILLATION COLUMNS HANDLING THREE PHASE SYSTEMS By YUSUF HEKIM,, Bachelor of Science Middle East Technical University Ankara, Turkey 1971 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE December, 1973 OKLAHOMA STATE UNiVERSIT LIBRARY APR 10 1974 A SHORT-CUT METHOD FOR PREDICTING THE TOTAL REFLUX PERFORMANCE OF DISTILLATION COLUMNS HANDLING THREE PHASE SYSTEMS Thesis Approved: l~ Dean of the Graduate College 8'7721!t ii PREFACE This study was carried out to develop a short-cut method of pre dtcting the minimun number of theoretical trays in distillation columns operating on feeds which can exhibit partial miscibility. These systems are usually encountered in the organic chemical industry. The method was applied to one binary and two ternary systems which exhibit hetero geneous azeotropic behavior. The method was developed specifically for single feed, two product distillation columns; but it can be easily extended to describe the behavior of various complex column configura tions as multiple feeds and more than two products. This will lead to an increase in the computational time requirements. Description of the thermodynamic behavior of the systems handled was critical to the solution of this problem. The Renon Non Random Two Liquid method of predicting liquid phase activity coefficients coupled with the Prausnitz model for the vapor phase fugacity was found to be the most successful method for predicting the vapor-liquid-liquid equilibria constants. I would like to give my sincere appreciation to Prof.