Enzymes for Wine Fermentation: Current and Perspective Applications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Enzymes Handling/Processing
Enzymes Handling/Processing 1 Identification of Petitioned Substance 2 3 This Technical Report addresses enzymes used in used in food processing (handling), which are 4 traditionally derived from various biological sources that include microorganisms (i.e., fungi and 5 bacteria), plants, and animals. Approximately 19 enzyme types are used in organic food processing, from 6 at least 72 different sources (e.g., strains of bacteria) (ETA, 2004). In this Technical Report, information is 7 provided about animal, microbial, and plant-derived enzymes generally, and more detailed information 8 is presented for at least one model enzyme in each group. 9 10 Enzymes Derived from Animal Sources: 11 Commonly used animal-derived enzymes include animal lipase, bovine liver catalase, egg white 12 lysozyme, pancreatin, pepsin, rennet, and trypsin. The model enzyme is rennet. Additional details are 13 also provided for egg white lysozyme. 14 15 Chemical Name: Trade Name: 16 Rennet (animal-derived) Rennet 17 18 Other Names: CAS Number: 19 Bovine rennet 9001-98-3 20 Rennin 25 21 Chymosin 26 Other Codes: 22 Prorennin 27 Enzyme Commission number: 3.4.23.4 23 Rennase 28 24 29 30 31 Chemical Name: CAS Number: 32 Peptidoglycan N-acetylmuramoylhydrolase 9001-63-2 33 34 Other Name: Other Codes: 35 Muramidase Enzyme Commission number: 3.2.1.17 36 37 Trade Name: 38 Egg white lysozyme 39 40 Enzymes Derived from Plant Sources: 41 Commonly used plant-derived enzymes include bromelain, papain, chinitase, plant-derived phytases, and 42 ficin. The model enzyme is bromelain. -
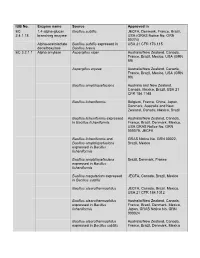
IUB No. Enzyme Name Source Approved in EC 1,4-Alpha-Glucan Bacillus Subtilis JECFA, Denmark, France, Brazil, 2.4.1.18 Branching Enzyme USA (GRAS Notice No
IUB No. Enzyme name Source Approved in EC 1,4-alpha-glucan Bacillus subtilis JECFA, Denmark, France, Brazil, 2.4.1.18 branching enzyme USA (GRAS Notice No. GRN 00274) Alpha-acetolactate Bacillus subtilis expressed in USA 21 CFR 173.115 decarboxylase Bacillus brevis EC 3.2.1.1 Alpha amylase Aspergillus niger Australia/New Zealand, Canada, France, Brazil, Mexico, USA (GRN 89) Aspergillus oryzae Australia/New Zealand, Canada, France, Brazil, Mexico, USA (GRN 90) Bacillus amyloliquefaciens Australia and New Zealand, Canada, Mexico, Brazil, USA 21 CFR 184.1148 Bacillus licheniformis Belgium, France, China, Japan, Denmark, Australia and New Zealand, Canada, Mexico, Brazil Bacillus licheniformis expressed Australia/New Zealand, Canada, in Bacillus licheniformis France, Brazil, Denmark, Mexico, USA GRAS Notice No. GRN 000079, JECFA Bacillus licheniformis and GRAS Notice No. GRN 00022, Bacillus amyloliquefaciens Brazil, Mexico expressed in Bacillus licheniformis Bacillus amyloliquefaciens Brazil, Denmark, France expressed in Bacillus licheniformis Bacillus megaterium expressed JECFA, Canada, Brazil, Mexico in Bacillus subtilis Bacillus stearothermophilus JECFA, Canada, Brazil, Mexico, USA 21 CFR 184.1012 Bacillus stearothermophilus Australia/New Zealand, Canada, expressed in Bacillus France, Brazil, Denmark, Mexico, licheniformis Japan, GRAS Notice No. GRN 000024 Bacillus stearothermophilus Australia/New Zealand, Canada, expressed in Bacillus subtilis France, Brazil, Denmark, Mexico Bacillus stearothermophilus Australia/New Zealand, Canada, (Geobacillus France, Europa, Brazil, Mexico, stearothermophilus) USA (GRN 594) Bacillus subtilis Australia and New Zealand, Canada, Mexico, Brazil, USA 21 CFR 184.1148 Rhizopus delemar Brazil Rhizopus oryzae Brazil, Mexico, USA GRAS Notice No. GRN 000090 Thermoccocales expressed in Brazil, USA (GRN126) Pseudomonas fluorecens Rhizomucor pusillus expressed Brazil, Denmark, France, Mexico, in Aspergillus niger Australia and New Zealand. -

Boards' Fodder
boards’ fodder Cosmeceuticals Contributed by Elisabeth Hurliman, MD, PhD; Jennifer Hayes, MD; Hilary Reich MD; and Sarah Schram, MD. INGREDIENT FUNCTION MECHANISM ASSOCIATIONS/SIDE EFFECTS Vitamin A/ Antioxidant (reduces free Affects gene transcription Comedolysis epidermal thickening, dermal Derivatives (retinal, radicals, lowers concentration differentiation and growth of regeneration, pigment lightening retinol, retinoic of matrix metalloproteinases cells in the skin acid, provitamin reduces collagen degradation) Side effects: Irritation, erythema, desquamation A, asthaxanthin, Normalizes follicular Elisabeth Hurliman, lutein) epithelial differentiation and keratinization MD, PhD, is a PGY-4 dermatology resident Vitamin C (L Secondary endogenous Ascorbic acid: necessary L-ascorbic acid + alpha-tocopherol (vitamin E)= ascorbic acid, antioxidant in skin cofactor for prolylhydroxylase UVA and UVB protection at University of tetrahexyldecyl and lysyl hydroxylase Minnesota department ascorbate) Lightens pigment Zinc, resveratrol, L-ergothioneine and tyrosine add of dermatology. (affects melanogenesis) L-ascorbic acid: scavenges to vitamin C bioavailability free oxygen radicals, Protects Vitamin E from oxidation stimulates collagen synthesis Improves skin texture and hydration May interrupt melanogenesis by interacting with copper ions Vitamin E/ Primary endogenous antioxidant Prevents lipid peroxidation; Alpha tocopherol is the most physiologically Tocopherols, in skin scavenges free oxygen active isomer Jennifer Hayes, MD, Tocotrienols -

Concepts in Wine Chemistry
THIRD EDITION Concepts IN Wine Chemistry YAIR MARGALIT, Ph.D. The Wine Appreciation Guild San Francisco Contents Introduction ix I. Must and Wine Composition 1 A. General Background 3 B. Sugars 5 C. Acids 11 D. Alcohols 22 E. Aldehydes and Ketones 30 F. Esters 32 G. Nitrogen Compounds 34 H. Phenols 43 I. Inorganic Constituents 52 References 55 n. Fermentation 61 A. General View 63 B. Chemistry of Fermentation 64 C. Factors Affecting Fermentation 68 D. Stuck Fermentation 77 E. Heat of Fermentation 84 F. Malolactic Fermentation 89 G. Carbonic Maceration 98 References 99 v III. Phenolic Compounds 105 A. Wine Phenolic Background 107 B. Tannins 120 C. Red Wine Color 123 D. Extraction of Phenolic Compounds from Grapes 139 References 143 IV. Aroma and Flavor 149 A. Taste 151 B. Floral Aroma 179 C. Vegetative Aroma 189 D. Fruity Aroma 194 E. Bitterness and Astringency 195 F. Specific Flavors 201 References 214 V. Oxidation and Wine Aging 223 A. General Aspects of Wine Oxidation 225 B. Phenolic Oxidation 227 C. Browning of White Wines 232 D. Wine Aging 238 References 253 VI. Oak Products 257 A. Cooperage 259 B. Barrel Aging 274 C. Cork 291 References 305 vi VH. Sulfur Dioxide 313 A. Sulfur-Dioxide as Food Products Preservative 315 B. Sulfur-Dioxide Uses in Wine 326 References 337 Vm. Cellar Processes 341 A. Fining 343 B. Stabilization 352 C. Acidity Adjustment 364 D. Wine Preservatives 372 References 382 IX. Wine Faults 387 A. Chemical Faults 389 B. Microbiological Faults 395 C. Summary ofFaults 402 References 409 X. -

CHABLIS LES CLOS GRAND CRU 2017 100% Chardonnay Although I’Ve Said It Before, It’S Worth Repeating: Drouhin Is One of the Top Producers in Chablis
CHABLIS LES CLOS GRAND CRU 2017 100% Chardonnay Although I’ve said it before, it’s worth repeating: Drouhin is one of the top producers in Chablis. - WineReviewOnline.com The vineyard site is the largest and probably most famous Grand Cru, located between Valmur on the left and Blanchot on the right. The exposure is responsible for its generous and powerful character. It is the cradle of Chablis, already recognized by the medieval monks as a superb location for planting a vineyard. The term “Les Clos” (enclosure, in French) probably refers to the surrounding wall that they built to fence off the parcel. This wall is no longer in existence. At the end of the 19th Century the vineyard was devastated by the phylloxera disease. In the 1960’s, Robert Drouhin was one of the first Beaune propriétaires to bring it back to life. Vineyard site: • Soil: the Kimmeridgian limestone contains millions of tiny marine fossils embedded in a kind of whitish mortar which may have been once the bottom of the sea...hundreds of million years ago. • This marine origin gives the wines of Chablis their unique flavour. • Drouhin estate: 1.3 ha. (3.212 acres). Average age of the vines: 37 years. Viticulture: • Plantation density: 8,000 to 10,000 stocks/ha. • Pruning: double Guyot “Vallée de la Marne” (for its resistance to frost). • Yield: we aim for a lower yield in order to extract all possible nuances from the terroir. • Average yield at the Domaine: 43.2hl/ha (authorized for the appellation is now 54hl/ha). Ageing • Type: in oak barrel (0% new wood). -

Download Abstracts
JUNE 17–20, 2019 Napa Valley Marriott Hotel Technical Abstracts Napa, California USA 70 YEARS th NATIONAL Science: A Platform 70 for Progress CONFERENCE ASEV AMERICAN SOCIETY FOR ENOLOGY AND VITICULTURE 70 Technical Abstracts YEARS Oral Presentation Abstracts Wednesday, June 19 Enology—Phenolic Extraction ......................................................................................................................48–50 Viticulture—Impact of Red Blotch on Grape and Wine Composition ............................................ 51–54 Science: A Platform A Platform Science: Progress for Enology—Microbiology of Wine .................................................................................................................. 54–56 Viticulture—Managing Pests and Weeds ..................................................................................................57–59 Enology—Wine Chemistry: Oxidations and Aging .................................................................................59-61 Viticulture—Fruit Composition and Yield ..................................................................................................61-63 Thursday, June 20 Enology—Wine Macromolecules ........................................................................................................................ 64 Viticulture—Crop Load Management .........................................................................................................65-66 Enology—Wine Stability ................................................................................................................................ -

(12) Patent Application Publication (10) Pub. No.: US 2016/0346364 A1 BRUNS Et Al
US 2016.0346364A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0346364 A1 BRUNS et al. (43) Pub. Date: Dec. 1, 2016 (54) MEDICAMENT AND METHOD FOR (52) U.S. Cl. TREATING INNATE IMMUNE RESPONSE CPC ........... A61K 38/488 (2013.01); A61K 38/482 DISEASES (2013.01); C12Y 304/23019 (2013.01); C12Y 304/21026 (2013.01); C12Y 304/23018 (71) Applicant: DSM IPASSETS B.V., Heerlen (NL) (2013.01); A61K 9/0053 (2013.01); C12N 9/62 (2013.01); A23L 29/06 (2016.08); A2ID 8/042 (72) Inventors: Maaike Johanna BRUINS, Kaiseraugst (2013.01); A23L 5/25 (2016.08); A23V (CH); Luppo EDENS, Kaiseraugst 2002/00 (2013.01) (CH); Lenneke NAN, Kaiseraugst (CH) (57) ABSTRACT (21) Appl. No.: 15/101,630 This invention relates to a medicament or a dietary Supple (22) PCT Filed: Dec. 11, 2014 ment comprising the Aspergillus niger aspergilloglutamic peptidase that is capable of hydrolyzing plant food allergens, (86). PCT No.: PCT/EP2014/077355 and more particularly, alpha-amylase/trypsin inhibitors, thereby treating diseases due to an innate immune response S 371 (c)(1), in humans, and/or allowing to delay the onset of said (2) Date: Jun. 3, 2016 diseases. The present invention relates to the discovery that (30) Foreign Application Priority Data the Aspergillus niger aspergilloglutamic peptidase is capable of hydrolyzing alpha-amylase/trypsin inhibitors that are Dec. 11, 2013 (EP) .................................. 13196580.8 present in wheat and related cereals said inhibitors being strong inducers of innate immune response. Furthermore, Publication Classification the present invention relates to a method for hydrolyzing alpha-amylase/trypsin inhibitors comprising incubating a (51) Int. -

Use of Plackett-Burman Design for Rapid Screening of Diverse Raw Pectin Sources for Cold-Active Polygalacturonase and Amylase Production by Geotrichum Sp
Int.J.Curr.Microbiol.App.Sci (2015) 4(6): 821-827 ISSN: 2319-7706 Volume 4 Number 6 (2015) pp. 821-827 http://www.ijcmas.com Original Research Article Use of Plackett-Burman Design for rapid Screening of Diverse Raw Pectin Sources for Cold-Active Polygalacturonase and Amylase Production by Geotrichum sp K. Divya and P. Naga Padma* Bhavan s Vivekananda College of Science, Humanities and Commerce, Secunderabad 94, India *Corresponding author A B S T R A C T Cold active polygalacturonases and amylases play significant role in extraction and clarification of fruit juices at industrial level. An optimized production medium with low cost substrates would be very useful for commercial production of these enzymes. The present study was done to screen low cost pectin and starch K e y w o r d s substrates for cold active enzymes production. The different pectin and starch sources screened were fruit and vegetables peels like citrus, pineapple, apple, Amylase, banana, mango, guava, carrot, beetroot, bottle gourd, ridge gourd and potato. For Cold-active efficient screening of the best sources a statistical design like Plackett-Burman was enzyme, used as in this design n variables can be studied in just n-1 experiments only. A Geotrichum sps, twelve experimental design Plackett-Burman was used as the best sources can be Poly shortlisted in consideration with their interactive effects. The pectinolytic and galacturonase, amylolytic yeast isolate was identified as Geotrichum sps and was used for the Plackett- present study. Cold-active pectinase and amylase enzyme activity was assayed by Burman, dinitrosalicylic acid (DNS) method. -

Oregon Wine Research Institute Viticulture & Enology Technical Newsletter Spring 2019
OREGON WINE RESEARCH INSTITUTE VITICULTURE & ENOLOGY TECHNICAL NEWSLETTER SPRING 2019 Welcome to the Spring 2019 Newsletter This edition contains research updates and a comprehensive list of In this issue: publications summarizing research conducted by faculty of the Oregon • Cluster thinning research: Wine Research Institute at Oregon State University. Dr. Patty Skinkis, OSU What are the limits? Viticulture Extension Specialist and Professor, opens the newsletter with • Effective microbial an article on canopy yield management. Dr. James Osborne, OSU Enology monitoring is key to Extension Specialist and Associate Professor, discusses the importance of preventing microbial spoilage effective microbial monitoring in preventing microbial spoilage. Lastly, Sarah • Fits like a glove: Improving Lowder, OSU Graduate Research Assistant, along with Dr. Walt Mahaffee, sampling techniques to Research Plant Pathologist, USDA-ARS, provide an article on techniques to monitor Qol fungicide monitor Qol fungicide resistant grape powdery mildew. resistant grape powdery This issue is posted online at the OWRI website https://owri.oregonstate. mildew edu/owri/extension-resources/owri-newsletters. Learn more about our • Research Publications research and engage with the core faculty here. Cheers, The OWRI Team Editorial Team Crop thinning research: What are the limits? Denise L. Dewey Dr. Patty Skinkis, Viticulture Extension Specialist and Professor, OSU Lead Editor Oregon Wine Research Institute [email protected] The yield-quality paradigm has long driven vineyard management decision- making, with growers focusing on the level of cluster thinning needed to Dr. Patty Skinkis reach target yields. The general thought is that reducing yield will improve Viticulture Editor ripeness and quality, allowing the remaining fruit to accumulate desirable Oregon Wine Research Institute [email protected] aroma, flavor, and color compounds. -

Copyrighted Material
1 Water and Ethanol 1.1 Introduction From a macroscopic perspective, wine is a mildly acidic hydroethanolic solution. As shown in Table 1.1, water and ethanol represent ~97% w/w of dry table wines. Ethanol is the major bioactive compound in wine and its presence renders wine and other alcoholic beverages inhospitable to microbial pathogens. Understanding the physiochemical properties of wine will first require a review of the basic properties of water and water–ethanol mixtures. More thorough discussions of the unique properties of water, including those specific to the food chemistry, can be found elsewhere [1]. 1.2 Chemical and physical properties of water Water is a hydride of oxygen, but has unique properties compared to other hydrides of elements nearby on the periodic table, as shown in Table 1.2. For example, the boiling point of water (100 °C) is far above that of hydrides of adjacent elements on the periodic table: HF (19.5 °C), H2S (–60 °C), and NH3 (–33 °C). Thus, water exists as a liquid at room temperature, while the other hydrides exist as gases. Similarly, water also has a higher heat of vaporization, heat capacity, and freezing point than would be expected as compared to nearby hydrides. The unique properties of water are largely due to its ability to engage in intermolecular hydrogen (H) bond- ing, which results in stronger molecule‐to‐molecule interactions than in related compounds. ●● Oxygen is more electronegativeCOPYRIGHTED than hydrogen and an O–H MATERIALbond is more polarized than N–H or S–H. ●● The geometry and symmetry of an H2O molecule allows for four concurrent H bonds per water molecule. -

PRODUCT INFORMATION Arbutin Item No
PRODUCT INFORMATION Arbutin Item No. 26407 CAS Registry No.: 497-76-7 Formal Name: 4-hydroxyphenyl β-D-glucopyranoside OH Synonyms: β-Arbutin, NSC 4036 MF: C H O OH 12 16 7 HO FW: 272.3 O Purity: ≥98% UV/Vis.: λmax: 221, 283 nm OH O Supplied as: A crystalline solid Storage: -20°C OH Stability: ≥2 years Information represents the product specifications. Batch specific analytical results are provided on each certificate of analysis. Laboratory Procedures Arbutin is supplied as a crystalline solid. A stock solution may be made by dissolving the arbutin in the solvent of choice. Arbutin is soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide, which should be purged with an inert gas. The solubility of arbutin in these solvents is approximately 1, 10, and 20 mg/ml, respectively. Further dilutions of the stock solution into aqueous buffers or isotonic saline should be made prior to performing biological experiments. Ensure that the residual amount of organic solvent is insignificant, since organic solvents may have physiological effects at low concentrations. Organic solvent-free aqueous solutions of arbutin can be prepared by directly dissolving the crystalline solid in aqueous buffers. The solubility of arbutin in PBS, pH 7.2, is approximately 3 mg/ml. We do not recommend storing the aqueous solution for more than one day. Description Arbutin is a glycosylated hydroquinone that has been found in Arctostaphylos plants and has diverse biological activities, including tyrosinase inhibitory, antioxidant, and anti-inflammatory properties.1,2 It inhibits human tyrosinase activity in crude tyrosinase solution isolated from human melanocytes (IC50s = 5.7 and 18.9 mM using L-tyrosine and L-DOPA as substrates, respectively) as well as in intact 3 melanocytes (IC50 = 0.5 mM). -

Progress in the Field of Aspartic Proteinases in Cheese Manufacturing
Progress in the field of aspartic proteinases in cheese manufacturing: structures, functions, catalytic mechanism, inhibition, and engineering Sirma Yegin, Peter Dekker To cite this version: Sirma Yegin, Peter Dekker. Progress in the field of aspartic proteinases in cheese manufacturing: structures, functions, catalytic mechanism, inhibition, and engineering. Dairy Science & Technology, EDP sciences/Springer, 2013, 93 (6), pp.565-594. 10.1007/s13594-013-0137-2. hal-01201447 HAL Id: hal-01201447 https://hal.archives-ouvertes.fr/hal-01201447 Submitted on 17 Sep 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Dairy Sci. & Technol. (2013) 93:565–594 DOI 10.1007/s13594-013-0137-2 REVIEW PAPER Progress in the field of aspartic proteinases in cheese manufacturing: structures, functions, catalytic mechanism, inhibition, and engineering Sirma Yegin & Peter Dekker Received: 25 February 2013 /Revised: 16 May 2013 /Accepted: 21 May 2013 / Published online: 27 June 2013 # INRA and Springer-Verlag France 2013 Abstract Aspartic proteinases are an important class of proteinases which are widely used as milk-coagulating agents in industrial cheese production. They are available from a wide range of sources including mammals, plants, and microorganisms.