Gruber, S., J.K. Nolan, and D. Reed. 2008
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Town of Chester CPP Plan 3-26-19
Community Preservation Plan Town of Chester, NY March 26, 2019 Committee Draft Prepared by the Town of Chester Community Preservation Plan Committee Prepared with technical assistance from: Planit Main Street, Inc. Preface The Town of Chester has long recognized that community planning is an ongoing process. In 2015, the Town Board adopted a Comprehensive Plan, which was an update of its 2003 Comprehensive Plan. The 2015 Comprehensive Plan recommended additional actions, plans and detailed studies to pursue the recommendations of the Comprehensive Plan. Among these were additional measures to protect natural resources, agricultural resources and open space. In September 2017, the Town Board appointed a Community Preservation Plan Committee (CPPC) to guide undertake the creation of the Town’s first Community Preservation Plan. This Community Preservation Plan is not a new departure - rather it incorporates and builds upon the recommendations of the Town’s adopted 2015 Comprehensive Plan and its existing land use regulations. i Acknowledgements The 2017 Community Preservation Plan (CPP) Steering Committee acknowledges the extraordinary work of the 2015 Comprehensive Plan Committee in creating the Town’s 2015 Comprehensive Plan. Chester Town Board Hon. Alex Jamieson, Supervisor Robert Valentine - Deputy Supervisor Brendan W. Medican - Councilman Cynthia Smith - Councilwomen Ryan C. Wensley – Councilman Linda Zappala, Town Clerk Clifton Patrick, Town Historian Town of Chester Community Preservation Plan Committee (CPPC) NAME TITLE Donald Serotta Chairman Suzanne Bellanich Member Tim Diltz Member Richard Logothetis Member Tracy Schuh Member Robert Valentine Member Consultant Alan J. Sorensen, AICP, Planit Main Street, Inc. ii Contents 1.0 Introduction, Purpose and Summary .............................................................................................. 4 2.0 Community Preservation Target Areas, Projects, Parcels and Priorities ..................................... -

Report: 13% of Hudson Valley Bridges in Poor Condition
Report: 13% of Hudson Valley bridges in poor condition FRONT PAGE lower-weight vehicles, that can have an impact on everyone from emergency responders to school buses, commercial vehicles and farm equipment, who will all need to make longer trips to get The Mill Street bridge, over Quassaick Creek in where they need to Newburgh/New Windsor, is the second-worst go. ranked bridge among the 25 cited in the Hudson Valley as poor/structurally deficient [KEELY “Good MARSH/FOR THE TIMES HERALD-RECORD] infrastructure is also the cornerstone of By Michael Randall bringing in good Times Herald-Record jobs,” said Mike GOSHEN – A new report from a nonprofit group Oates, president that researches and evaluates the conditions of and CEO of Hudson the roads we travel on says 13 percent of the Valley Economic Hudson Valley’s bridges are in poor, structurally Development deficient condition. Corporation. “I hope Another 64 percent are in fair condition, while this (report) leads to only 23 percent are in good condition. action, so we’re not At a news conference Tuesday at the Orange at a competitive County Chamber of Commerce office, Carolyn disadvantage with Bonifas Kelly, associate director of research for other states.” the nonprofit, The Road Information Project New York ranks (TRIP), said those structurally deficient bridges 12th among the 50 6. Pine Hill Road over the Thruway, Woodbury carry almost 2.6 million vehicles per day. states, with 10 percent of its bridges in poor or 12. South Street over Wawayanda Creek, Warwick Her study included 2,551 bridges in Orange, structurally deficient condition. -

Public Road Scenic Alternative Phasing
Figure 4: Public Road Scenic Alternative Phasing Plan BT Holdings - Chester Development Village of Chester, Town of Chester, Orange County, New York Source: Langan Engineering & Environmental Services, 08/18/11 Scale: As shown File 05009 08/26/11 JS/05009 Tim Miller Associates, Inc.,10 North Street, Cold Spring, New York 10516 (845) 265-4400 Fax (845) 265-4418 Project Description August 18, 2011 2.0 DESCRIPTION OF PROPOSED ACTION COMMENTS AND RESPONSES Comment 2.0-1 (Public Hearing, January 7, 2010, Steven Neuhaus, Town of Chester Town Supervisor): On August 10th, 2009, I sent a letter on behalf of the Town to the Village of Chester regarding our DEIS comments. We feel that there are at least twenty comments that weren't answered as thoroughly, or to our liking. I'd like to suggest or recommend that the BT Holdings engineers and architects or planners sit down with the Town's engineers and consultants, and just go through them item by item, so they are thoroughly explained and looked at. Response 2.0-1: During the course of the environmental review of the BT Holdings project, the Village of Chester circulated a notice of intent to be lead agency which the Town of Chester acknowledged. However, due to the nature of this specific project, the Village of Chester went above and beyond the measures normally taken to insure that a coordinated review of this project was conducted. To that end, in order to have benefit of the Town's comments as early in the process as possible, the Village gave the Town a preliminary working copy of the DEIS prior to its being accepted as complete by the Village. -

Sussex County Open Space and Recreation Plan.”
OPEN SPACE AND RECREATION PLAN for the County of Sussex “People and Nature Together” Compiled by Morris Land Conservancy with the Sussex County Open Space Committee September 30, 2003 County of Sussex Open Space and Recreation Plan produced by Morris Land Conservancy’s Partners for Greener Communities team: David Epstein, Executive Director Laura Szwak, Assistant Director Barbara Heskins Davis, Director of Municipal Programs Robert Sheffield, Planning Manager Tanya Nolte, Mapping Manager Sandy Urgo, Land Preservation Specialist Anne Bowman, Land Acquisition Administrator Holly Szoke, Communications Manager Letty Lisk, Office Manager Student Interns: Melissa Haupt Brian Henderson Brian Licinski Ken Sicknick Erin Siek Andrew Szwak Dolce Vieira OPEN SPACE AND RECREATION PLAN for County of Sussex “People and Nature Together” Compiled by: Morris Land Conservancy a nonprofit land trust with the County of Sussex Open Space Advisory Committee September 2003 County of Sussex Board of Chosen Freeholders Harold J. Wirths, Director Joann D’Angeli, Deputy Director Gary R. Chiusano, Member Glen Vetrano, Member Susan M. Zellman, Member County of Sussex Open Space Advisory Committee Austin Carew, Chairperson Glen Vetrano, Freeholder Liaison Ray Bonker Louis Cherepy Libby Herland William Hookway Tom Meyer Barbara Rosko Eric Snyder Donna Traylor Acknowledgements Morris Land Conservancy would like to acknowledge the following individuals and organizations for their help in providing information, guidance, research and mapping materials for the County of -

LOCAL IMPACTS and COSTS Exhibit IX. A.2.B Traffic and Roadway
LOCAL IMPACTS AND COSTS Exhibit IX. A.2.b The proposed Resorts World Hudson Valley is a mixed use development that will incorporate a 600‐room hotel with a full‐service gaming facility and a conference center, along with associated, complementary amenities on an approximate 373‐acre site. Potential impacts include those to traffic and noise, watershed impacts from stormwater and wastewater discharge, and hydrologic impacts to surface waters and wetlands. The design for Resorts World Hudson Valley has been developed in conjunction with LEED® measures to minimize impacts to the greatest extent possible. Where impacts cannot be avoided, RW Orange County LLC has developed a cohesive mitigation strategy as detailed in Exhibit IX.A.3 Mitigation of Impact to Host Municipality and Nearby Municipalities. Traffic and Roadway Infrastructure Preliminary Transportation Demand Impacts Development of the proposed Resorts World Hudson Valley would generate substantial numbers of new vehicular trips by auto, taxi and bus on the roadway system providing access to the project site. The majority of these trips (approximately 90 percent) would arrive and depart via I‐84, with Route 17K, Route 747 and Route 207 providing local access. Most vehicles destined to/from I‐84 would use the I‐84/Route 747 interchange located immediately to the southeast of the project site which was designed to accommodate future demand from expanded use of the nearby Stewart International Airport. As this airport demand has not been realized, the I‐84/Route 747 interchange typically functions with available capacity during peak periods. A new signalized intersection on Route 17K and a new roundabout on Route 747 would provide access to the proposed project’s internal roadway system. -
NY Excluding Long Island 2017
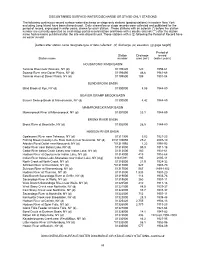
DISCONTINUED SURFACE-WATER DISCHARGE OR STAGE-ONLY STATIONS The following continuous-record surface-water discharge or stage-only stations (gaging stations) in eastern New York excluding Long Island have been discontinued. Daily streamflow or stage records were collected and published for the period of record, expressed in water years, shown for each station. Those stations with an asterisk (*) before the station number are currently operated as crest-stage partial-record station and those with a double asterisk (**) after the station name had revisions published after the site was discontinued. Those stations with a (‡) following the Period of Record have no winter record. [Letters after station name designate type of data collected: (d) discharge, (e) elevation, (g) gage height] Period of Station Drainage record Station name number area (mi2) (water years) HOUSATONIC RIVER BASIN Tenmile River near Wassaic, NY (d) 01199420 120 1959-61 Swamp River near Dover Plains, NY (d) 01199490 46.6 1961-68 Tenmile River at Dover Plains, NY (d) 01199500 189 1901-04 BLIND BROOK BASIN Blind Brook at Rye, NY (d) 01300000 8.86 1944-89 BEAVER SWAMP BROOK BASIN Beaver Swamp Brook at Mamaroneck, NY (d) 01300500 4.42 1944-89 MAMARONECK RIVER BASIN Mamaroneck River at Mamaroneck, NY (d) 01301000 23.1 1944-89 BRONX RIVER BASIN Bronx River at Bronxville, NY (d) 01302000 26.5 1944-89 HUDSON RIVER BASIN Opalescent River near Tahawus, NY (d) 01311900 9.02 1921-23 Fishing Brook (County Line Flow Outlet) near Newcomb, NY (d) 0131199050 25.2 2007-10 Arbutus Pond Outlet -

Army Corps of Engineers Response Document Draft
3.0 ORANGE COUNTY Orange County has experienced numerous water resource problems along the main stem and the associated tributaries of the Moodna Creek and the Ramapo River that are typically affected by flooding during heavy rain events over the past several years including streambank erosion, agradation, sedimentation, deposition, blockages, environmental degradation, water quality and especially flooding. However, since October 2005, the flooding issues have severely increased and flooding continues during storm events that may or may not be considered significant. Areas affected as a result of creek flows are documented in the attached trip reports (Appendix D). Throughout the Orange County watershed, site visits confirmed opportunities to stabilize the eroding or threatened banks restore the riparian habitat while controlling sediment transport and improving water quality, and balance the flow regime. If the local municipalities choose to request Federal involvement, there are several options, depending on their budget, desired timeframe and intended results. The most viable options include a specifically authorized watershed study or program, or an emergency streambank protection project (Section 14 of the Continuing Authorities Program), or pursing a Continuing Authorities Program study for Flood Risk Management or Aquatic Ecosystem Restoration (Section 205 and Section 206 of the Continuing Authorities Program, respectively). Limited Federal involvement could also be provided in the form of the Planning Assistance to States or Support for Others programs provide assistance and limited funds outside of traditional Corps authorities. A watershed study focusing on restoration of the Moodna Creek, Otter Creek, Ramapo River and their associated tributaries could address various problems using a systematic approach. -
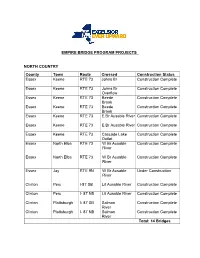
Empire Bridge Program Projects North Country
EMPIRE BRIDGE PROGRAM PROJECTS NORTH COUNTRY County Town Route Crossed Construction Status Essex Keene RTE 73 Johns Br Construction Complete Essex Keene RTE 73 Johns Br Construction Complete Overflow Essex Keene RTE 73 Beede Construction Complete Brook Essex Keene RTE 73 Beede Construction Complete Brook Essex Keene RTE 73 E Br Ausable River Construction Complete Essex Keene RTE 73 E Br Ausable River Construction Complete Essex Keene RTE 73 Cascade Lake Construction Complete Outlet Essex North Elba RTE 73 W Br Ausable Construction Complete River Essex North Elba RTE 73 W Br Ausable Construction Complete River Essex Jay RTE 9N W Br Ausable Under Construction River Clinton Peru I-87 SB Lit Ausable River Construction Complete Clinton Peru I- 87 NB Lit Ausable River Construction Complete Clinton Plattsburgh I- 87 SB Salmon Construction Complete River Clinton Plattsburgh I- 87 NB Salmon Construction Complete River Total: 14 Bridges CAPITAL DISTRICT County Town Route Crossed Construction Status Warren Thurman Rte 28 Hudson River Construction Complete Washington Hudson Falls Rte 196 Glens Falls Construction Complete Feeder Canal Washington Hudson Falls Rte 4 Glens Falls Construction Complete Feeder Saratoga Malta Rte 9 Kayaderosseras Construction Complete Creek Saratoga Greenfield Rte 9n Kayaderosseras Construction Complete Creek Rensselaer Nassau Rte 20 Kinderhook Creek Construction Complete Rensselaer Nassau Rte 20 Kinderhook Creek Construction Complete Rensselaer Nassau Rte 20 Kinderhook Creek Construction Complete Rensselaer Hoosick Rte -

SPDES Multi-Sector General Permit (MSGP) Facilities
SPDES Multi-Sector General Permit (MSGP) Facilities Status DEC Region NPDES ID Terminated 8 NYR00B679 Terminated 4 NYR00E979 Terminated 4 NYR00E094 Terminated 3 NYR00F907 Terminated 4 NYR00F128 Terminated 3 NYR00F294 Active 4 NYR00F440 Terminated 5 NYR00E193 Active 8 NYR00F759 No Exposure 1 NYR00G254 No Exposure 2 NYR00F729 Active 4 NYR00B055 Active 4 NYR00B054 No Exposure 8 NYR00G118 Active 3 NYR00B923 No Exposure 3 NYR00E656 Terminated 2 NYR00D894 No Exposure 1 NYR00F797 Active 3 NYR00B036 Page 1 of 1078 09/28/2021 SPDES Multi-Sector General Permit (MSGP) Facilities Name of Facility Location of Facility J A YANSICK LUMBER CO STATE RTE 70 LOCHVUE SPRING AVE EXT MAIN BROTHERS OIL CO INC - ROXBURY TERMINAL 25 LOCUST ST MONDELEZ GLOBAL LLC - NEWBURGH 800 CORPORATE BLVD TRAVIS 8412 STATE HWY 7 WASSAIC PIT BOX 221A P&M BRICK LLC MARINE TERMINAL 2170 RIVER RD CLINTON QUARRY LOST NATION RD ELMIRA ROAD MATERIALSLLC 1 COUNTY ROUTE 77A AEROFLEX PLAINVIEW 35 S. SERVICE RD. PRATT INSTITUTE MANHATTAN CAMPUS OPERATIONS 142-144 W 14TH ST WEST SAND LAKE PIT 3600 STATE RTE 43 HEMSTREET PARK BANK 3040 RIVER ROAD LOVE BEETS 1150 LEE RDSECT A FIRST STUDENT INC #12370 32 FITCHETT WAY UNITED STATES MINT NYS RTE 218 SWING STAGING INCORPORATED 55-51 43RD ST L-3 NARDA-ATM 49 RIDER AVE JOSEPH Y. RESNICK AIRPORT 199 AIRPORT ROAD Page 2 of 1078 09/28/2021 SPDES Multi-Sector General Permit (MSGP) Facilities City of Facility Zip of Facility County Name Sector Code HUNT 14846 Livingston POESTENKILL 12140 Rensselaer ROXBURY 12474 Delaware NEWBURGH 12550 Orange MARYLAND -

A Bibliography of the Wallkill River Watershed
wallkill river watershed alliance we fight dirty A Bibliography of the Wallkill River Watershed Many of the documents listed below will eventually be found in the documents section of the Wallkill River Watershed Alliance’s website at www.wallkillalliance.org/files Amendment to the Sussex County Water Quality Management Plan, Total Maximum Daily Load to Address Arsenic in the Wallkill River and Papakating Creek, Northwest Water Region. (2004). New Jersey Department of Environmental Protection, Division of Watershed Management, Bureau of Environmental Analysis and Restoration. Barbour, J., G. (undated manuscript). Ecological issues of Glenmere Lake, Town of Warwick, New York. Barringer, J. L., Bonin, J. L., Deluca, M. J., Romagna, T., Cenno, K., Marzo, A., Kratzer, T., Hirst, B. (2007). Sources and temporal dynamics of arsenic in a New Jersey watershed, USA. Science of the Total Environment, 379, 56-74. Barringer, J. L., Wilson, T. P., Szabo, Z., Bonin, J. L., Fischer, J. M., Smith, N. P., (2008). Diurnal variations in, and influences on, concentrations of particulate and dissolved arsenic and metals in the mildly alkaline Wallkill River, New Jersey, USA. Environmental Geology, 53, 1183-1199. Bugliosi, E. F., Casey, G. D., Ramelot, D. (1998). Geohydrology and water quality of the Wallkill River valley near Middletown, New York. United States Geological Survey, Open File Report 97-241. Dwaar Kill, Lower and Tribs Fact Sheet. (2007). Waterbody Inventory/Priority Waterbodies List. New York State Department of Environmental Conservation, Division of Water. Dwaar Kill, and Tribs Fact Sheet. (2007). Waterbody Inventory/Priority Waterbodies List. New York State Department of Environmental Conservation, Division of Water. -

REVISED UPDATED COMPREHENSIVE PLAN for the TOWN of GOSHEN Goshen, New York
REVISED UPDATED COMPREHENSIVE PLAN FOR THE TOWN OF GOSHEN Goshen, New York PUBLIC HEARING DRAFT Prepared for: Town of Goshen July 17, 2008 PUBLIC HEARING DRAFT REVISED COMPREHENSIVE PLAN Town of Goshen, New York 41 Webster Avenue Goshen, NY 10924 Prepared by: Town of Goshen Town Board Douglas Bloomfield, Supervisor Kenneth Newbold Louis Cappella George Lyons Philip Canterino Legal Counsel Dennis Caplicki, Attorney for the Town Richard B. Golden, Planning Board Attorney Kelly M. Naughton, Planning Board Attorney And Edwin Garling, Planner for the Town Prepared with the assistance of: BFJ Planning: Frank Fish, FAICP, Principal Georges Jacquemart, Principal Sarah K. Yackel, AICP, Associate Alireza Rabiee, Senior Transportation Planner Áine Balfe, Planner T. 212.353.7474 And Riddick Associates, P.C. Dennis Lindsay, Town Consulting Engineer T. 845.357.7238 July 17, 2008 Updated Town of Goshen Comprehensive Plan, 2008 TABLE OF CONTENTS 1.0 Introduction 1 1.0 Introduction 1 1.1 What is a Comprehensive Plan? 2 1.2 Goals and Objectives 4 1.3 The Planning Process 4 1.4 Planning in Goshen 5 1.5 Regional Planning Context 11 2.0 Existing Conditions 16 2.1 Geography 16 2.2 Water Supply 16 2.3 Political Structure 18 2.4 Demographics 23 2.5 Existing Land Uses 26 2.6 Review of Existing Zoning 31 2.7 Traffic Analysis 34 3.0 Comprehensive Plan 51 3.1 Goals and Objectives 51 3.2 Key Elements 52 3.3 Land Use Recommendations 55 3.4 Open Space and Recreation Plan 58 3.5 Economic Plan 64 3.6 Other Plan Recommendations 66 4.0 Implementation 67 4.1 State Environmental -

Appendix-J-JCMUA-SIAR-092520.Pdf
Passaic Valley Sewerage Commission October 2020 PVSC Treatment District Regional Long Term Control Plan Appendix J Selection and Implementation of Alternatives Report for Jersey City MUA Jersey City Municipal Utilities Authority Selection and Implementation of Alternatives Report NJPDES Permit No. NJ0108723 March 2020 Jersey City Municipal Utilities Authority January 2020 Selection and Implementation of Alternatives Report Page 1 of 45 TABLE OF CONTENTS SECTION A - INTRODUCTION .................................................................................................. 4 SECTION B - SCREENING OF CSO CONTROL TECHNOLOGIES ........................................ 9 SECTION C - EVALUATION OF ALTERNATIVES................................................................ 10 INTRODUCTION ......................................................................................................... 10 DEVELOPMENT AND EVALUATION OF ALTERNAtIVES.................................. 11 SECTION D - SELECTION OF RECOMMENDED LTCP ....................................................... 12 INTRODUCTION ......................................................................................................... 12 LTCP SELECTION PROCESS ..................................................................................... 12 SELECTION OF ALTERNATIVES ............................................................................. 12 D.3.1 Description ............................................................................................................... 13 D.3.2