Acid-Base Theories and Frontier Orbitals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter Outline
11/16/2016 Aqueous Equilibria: Chemistry of the Water World Chapter Outline • 15.1 Acids and Bases: The BrØnsted–Lowry Model • 15.2 Acid Strength and Molecular Structure • 15.3 pH and the Autoionization of Water • 15.4 Calculations Involving pH, Ka, and Kb • 15.5 Polyprotic Acids • 15.6 pH of Salt Solutions • 15.7 The Common-Ion Effect • 15.8 pH Buffers • 15.9 pH Indicators and Acid–Base Titrations • 15.10 Solubility Equilibria 2 1 11/16/2016 Acids Have a sour taste. Vinegar owes its taste to acetic acid. Citrus fruits contain citric acid. React with certain metals to produce hydrogen gas. React with carbonates and bicarbonates to produce carbon dioxide gas Bases Have a bitter taste. Feel slippery. Many soaps contain bases. Nomenclature Review – Ch 4, Section 4.2 You are only responsible for nomenclature taught in the lab. These ions are part of many different acids and you need to know them! 3- 2- - PO4 , HPO4 , H2PO4 H3PO4 2- - SO4 , HSO4 H2SO4 2- - SO3 , HSO3 H2SO3 2- - CO3 , HCO3 H2CO3 - - HNO HNO NO3 , NO2 3, 2 2- - S , HS H2S - - C2H3O2 (CH3COO ) HC2H3O2 binary acids, oxoacids HCl, HClO4 2 11/16/2016 Strong and Weak Acids A Brønsted acid is a proton donor A Brønsted base is a proton acceptor Strong Acid: Completely ionized - + HNO3(aq) + H2O(ℓ) → NO3 (aq) + H3O (aq) (H+ donor) (H+ acceptor) Weak Acid: Partially ionized - + HNO2(aq) + H2O(ℓ) ⇌ NO2 (aq) + H3O (aq) (H+ donor) (H+ acceptor) 3 11/16/2016 Hydronium Ion Conjugate Acid-Base Pairs 4 11/16/2016 Weak Acids reordered stronger − + CH3COO (aq) [H3O (aq)] Kc = CH3COOH(aq) [H2O(l)] 5 11/16/2016 Strong and Weak Bases Strong and Weak Bases 6 11/16/2016 Weak Bases 7 11/16/2016 Relative Strengths of Acids/Bases Leveling Effect: + • H3O is the strongest H+ donor that can exist in water. -

Unit 9 Neutralization Titrations-Ii
Estimations Based On Kinetic and Acid-Base UNIT 9 NEUTRALIZATION TITRATIONS-II Equilibria Studies Structure 9.1 Introduction Objectives 9.2 Non-aqueous Titrations 9.3 Role of Solvents in Acid-Base Reactions 9.4 Solvent Systems 9.5 Importance of Dielectric Constant 9.6 Hammett’s Acidity Functions 9.7 Titrants and End Point Detection 9.8 Some Applications 9.9 Summary 9.10 Terminal Questions 9.11 Answers 9.1 INTRODUCTION In the last unit you have learnt about the neutralization titrations in aqueous medium. I hope you know that water is poorly dissociated. But that does not prevent water to dissolve many of the electrolytes. Quantitative methods of analysis have been developed by these sort of rapid ionic reactions. It is economical and easier to handle aqueous solutions leading to the wide use of aqueous solution for analysis. But in many instances it is seen that non-aqueous ionizing solvents are advantageous in case of acidimetry and alkalimetry. This is specially true for cases where compounds cannot be titrated in an aqueous medium. In this unit I am going to introduce you to the non-aqueous titrations, the purpose of a particular solvent in specific reactions and also the various solvent systems. Have you heard about the term “dielectric constant”? Well in this unit you will also learn about its importance. After discussing Hammett’s acidity functions, titrants, end point detection, I will also bring to your notice certain applications for such titrations. This unit will help you to learn about the different aspects of nonaqueous neutralisation titrations which are utilised in analytical chemistry. -

WB Jensen, the Lewis Acid-Base Concepts (1980)
1 W. B. Jensen, The Lewis Acid-Base Concepts (1980) Most of this chapter is concerned with the Lewis definition, its more recent explanation in terms of molecular orbitals, & its application to inorganic chemistry. 2 6-1-1 History 3 6-2 Major Acid-Base Concepts 6-2-1 Arrhenius Concept Arrhenius received 1903 Nobel Prize in chemistry for this theory, Arrhenius acids form hydrogen ions (now frequently called hydronium or oxonium + ions, H3O ) in aqueous solution, Arrhenius bases form hydroxide ions in aqueous solution. 4 This explanation works well in aqueous solution, but it is inadequate for nonaqueous solutions & for gas & solid phase reactions in which H+ & OH- may not exist. Definition by Br∅nsted & Lewis are more appro- priate for general rule. 5 6-2-2 Br∅nsted-Lowry concept In 1923, Br∅nsted-Lowry defined an acid as a species with a tendency to lose a hydrogen ion & a base as a species with a tendency to gain a hydrogen ion. This definition expanded the Arrhenius list of acids & bases to include the gaseous HCl & NH3. 6 This definition also introduced the concept of conjugate acids & bases, differing only in the presence or absence of a proton, & described all reactions as occurring between a stronger acid & base to form a weaker acid & base. 7 Na + NH3 8 6-2-3 Solvent System Concept The solvent system definition applies to any solvent that can dissociate into a cation & an anion (autodissociation), where the cation resulting from autodissociation is the acid & the anion is the base. Solutes that increase the conc of the cation of the solvent are considered acids & solutes that increase the conc of the anion are considered bases. -

Chapter 15: Acids and Bases
CHAPTER 15: ACIDS AND BASES Part One: Acid-Base Concepts A. Properties of Aqueous Solutions of Acids. 1. Sour taste. (Examples: vinegar = acetic acid; lemons - citric acid) 2. Change the colors of many indicators: (pH-sensitive dyes) acid a. blue litmus → red acid b. bromothymol blue → yellow 3. React with active metals liberating H2(g). (HNO3 is exception, an oxidizing acid) Ca(s) + 2 HI(aq) → CaI2(aq) + H2(g) + 2+ 2 H (aq) + Zn(s) + 2 HNO3(aq) → Zn (aq) + 2 NO2(g) + 2 H2O(l) 4. Neutralize metal oxides and hydroxides to form salts and water: 2 HCl + MgO → MgCl2 + H2O 2 HCl + Ba(OH)2 → BaCl2 + H2O 5. React with salts of weaker acids to form the weaker acid and a new salt: HCl + NaF → NaCl + HF sodium hydrofluoric fluoride acid 6. Wholly or partially ionize in water and conduct electric current. B. Properties of Aqueous Solutions of Bases. 1. Bitter taste. (e.g. the bitter taste of soaps and many pharmaceuticals is because of their alkalinity) 2. Slippery feeling. (soaps are mildly alkaline) 3. Change the color of many indicators: Chapter 15 Page 1 base a. red litmus → blue base b. bromothymol blue yellow → blue 4. Neutralize protonic acids forming salts and water. • protonic acids - containing H+ also called “protic” 5. Ionize in water and conduct current. C. Arrhenius Theory. (1884) (Section 15.1) 1. An acid = substance that contains hydrogen and produces H+ in aqueous solution. • protonic acids - containing H+ also called “protic” acids 2. A base = substance that contains OH group and produces OH- ions in aqueous solution. -

Online Study Resource
Dr. A. P. J. Abdul Kalam Government College Department of Chemistry ONLINE STUDY RESOURCE For Detailed Discussion Contact Any Faculty Member Electronically Disclaimer: This Study Material is Collected From Available Materials in Internet and not a Copyrighted Property of this Institution. This Material is Intended to be Used for Purely Learning Purpose Without Any Fees or Charges Chapter 4. Acids and bases Brønsted acidity 111 4.1 Proton transfer equilibria in water 112 4.2 Solvent levelling 119 4.3 The solvent system de_nition of acids and bases 121 Characteristics of Brønsted acids 122 4.4 Periodic trends in aqua acid strength 122 4.5 Simple oxoacids 123 4.6 Anhydrous oxides 126 4.7 Polyoxo compound formation 127 4.8 Nonaqueous solvents 129 Lewis acidity 131 4.9 Examples of Lewis acids and bases 132 4.10 Group characteristics of Lewis acids 133 Reactions and properties of lewis acids and bases 136 4.11 The fundamental types of reaction 137 4.12 Hard and soft acids and bases 138 4.13 Thermodynamic acidity parameters 140 4.14 Solvents as acids and bases 141 Applications of acid–base chemistry 142 4.15 Superacids and superbases 142 4.16 Heterogeneous acid–base reactions 143 Chapter 5. Acids and bases Definitions of acids and bases: Arrhenius, Bronsted acidity, Lewis Arrhenius acid: generates [H+] in solution base: generates [OH-] in solution normal Arrhenius equation: acid + base <---> salt + water example: HCl + NaOH <---> NaCl + H2O Bronsted-Lowery:The Brønsted/Lowry Defintions specifies an acid as a proton donor and a base as a proton acceptor which applies to aqueous systems. -

Acid–Base and Donor– Acceptor Chemistry
Chapter 6 Acid–Base and Donor– Acceptor Chemistry 6.1 Acid–Base Models as Organizing Concepts A long-standing chemical objective is to organize reactions by using models to account for trends and gain insight into what properties of reactants are prerequisites for chemical change. Analyzing trends among similar reactions permits discovery of structure–function relationships (for example, how do molecular geometry and electronic structure influence reactivity?) and guides the design of molecules for practical use. Classifying substances as acids and bases has been important since ancient times; alchemists used neutralization—the ubiquitous reaction of an acid and base to form salt and water—to compile observations about different substances that engaged in similar reactions. Without modern structural analysis tools, such as X-ray crystallography and NMR spectroscopy, alchemists used their senses: they observed the tastes of acids (sour) and bases (bitter) and color changes of indicators. Many acid–base definitions have been devised, but only a few have been widely adopted. This chapter discusses the major acid–base models and their application in inorganic chemistry. After a historical introduction (Section 6.1.1), the models are presented in the rough order of their development. Among these are the ones attributed to Arrhenius (Section 6.2), Brønsted–Lowry (Section 6.3), and Lewis (Section 6.4). These sections emphasize the challenges associated with quantifying acidity and basicity, and rela- tionships between acid/base strength and molecular structure. The 1960s application of molecular orbitals (i.e., HOMO/LUMO interactions) to frame Lewis acid–base reactions (Section 6.4.1) permeates inorganic chemistry and dramatically expands the perspective on what constitutes an acid–base reaction. -
Introducing Acids and Bases
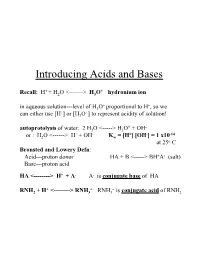
Introducing Acids and Bases + + Recall: H + H2O <-------> H3O hydronium ion + + in aqueous solution---level of H3O proportional to H , so we + + can either use [H ] or [H3O ] to represent acidity of solution! + - autoprotolysis of water: 2 H2O <-----> H3O + OH + - + - -14 or : H2O <------> H + OH Kw = [H ] [OH ] = 1 x10 at 25o C Bronsted and Lowery Defn: Acid---proton donor HA + B <-----> BH+A- (salt) Base---proton acid HA <--------> H+ + A- A- is conjugate base of HA + + + RNH2 + H <--------> RNH3 RNH3 is conjugate acid of RNH2 Consequences of Autoprotolysis rxn of Water: •can always find concentration of H+ or OH- if other species is known since in an aqueous solution---the autoprotolysis rxn is always in equilibrium!----product of [H+] [OH-] must equal 1 x10-14 + -3 - + -12 e.g.---if you know [H ] = 2 x 10 M; then [OH ] = Kw/ [H ] = 5 x 10 •in pure water---[H+] [OH-] = x2 = 10-14; Hence [H+] = [OH-] = 1 x 10-7 pH and [H+]; + + define pH = - log [H ] (we will see later that pH = -log aH ---but + + in dilute solutions---[H ] = aH ) if [H+] - 3.8 x 10-8 ; pH = - log(3.8 x 10-8) = 7.42 pH Scale pH = -log [H+] ; therefore: [H+] = 10-pH (can have pH values < 0 (negative #) and > 14--very strong acids/bases at very high concentrations) 0.1 M HCl strong acid--100% dissociation 0.001 M HCl • 0.00001 M HCl • What about 1x10-8 M HCl? pH=8 ?? – A dilute acid cannot be basic. We shall see--in a minute! pH for strong acid and strong base determined by moles of H+ or OH- generated by complete dissociation of these species; -3 + - 10 M HCl; HCl(aq) -

Teori Asam Basa
ASAL USUL TEORI ASAM BASA Lavoisier (1776) mengemukakan teori asam yang hanya terfokus pada asam oksi seperti HNO3 dan H2SO4. sedangkan asam-asam hidro halida tidak dapat didefinisikan Sir Humphry Davy (1810)memberikan istilah asam untuk senyawa hidrohalida meskiput kurang terstruktur ASAL USUL TEORI ASAM BASA Berzelius HanyaAsam berlakuBasa30 Senyawa-senyawatahunSenyawa-senyawa yang mengandung yang mengandung oksida non logam oksida logam ASAL USUL TEORI ASAM BASA Justus von Liebig (1838) HanyaAsam berlaku 50Basa tahun Zat-zat yang mengandung Zat-zat yang mengandung hidrogen yang tidak hidrogen yang dapat dapat digantikan dengan digantikan dengan logam logam TEORI ASAM BASA Secara Umum : Cairan berasa asam dan dapat Asam : memerahkan kertas lakmus biru Cairan berasa pahit dan Basa : dapat membirukan kertas lakmus merah Garam : Cairan yang berasa asin • Terdapat 7 teori Asam Basa yang masih dikenal : • Teori Arrhenius • Teori Bronstead-Lowry • Teori Lewis • Teori Pelarut • Teori Lux-Flood • Teori Usanovich • Teori Pearson TEORI ASAM BASA Teori Arrhenius (1887) Asam adalah senyawa yang melepaskan H+ dalam air. Contoh : Basa adalah senyawa yang melepaskan OH- dalam air Contoh : Kelemahan : hanya berlaku untuk larutan dalam air saja. TEORI ASAM BASA Teori Bronsted – Lowry(1923) Asam : Senyawa yg dapat memberikan proton ( H+ ) / donor proton. Basa: Senyawa yg dapat menerima proton (H+) / akseptor proton. Reaksi tanpa Pelarut Air + - HCl(g) + NH3(g) NH4 + Cl NH4Cl(s) Asam Basa Reaksi dengan Pelarut Air + - HCl(g) + H2O(aq) H3O (aq) + Cl -

Chapter 16. Acid-Base Equilibria Common Student Misconceptions • Students Often Confuse a Weak Acid with a Dilute Acid
Acid-Base Equilibria 231 Chapter 16. Acid-Base Equilibria Common Student Misconceptions • Students often confuse a weak acid with a dilute acid. • Students have problems with the numerical parts of this chapter. They should be strongly encouraged to do many problems on their own. • Students often have difficulty using their calculators to take decimal logarithms and antilogarithms. • Students confuse decimal (log) and natural (ln) logarithms. • Students have a difficult time accepting that acids may be neutral as well as both positively and negatively charged species. + – –14 • Students tend to forget that [H3O ][OH ] is equal to 1.0 × 10 only at 25 °C. • Students often wrestle with neutralization reactions producing solutions with pH different from 7. Teaching Tips • Determining pH of salts is often very conceptually and mathematically challenging. Solving and assigning many problems is needed to overcome these difficulties. • Students should be made aware of the fact that terms such as hydrogen ion, aqueous proton – or simply proton, as well as hydronium ion, are often used interchangeably in the context of acid-base chemistry. • Students should be reminded that water has both acidic and basic properties depending on what is mixed with it. • Leveraging students’ familiarity with Lewis Structures is useful in explaining relative strength of acids and reinforces ideas learned in Chapter 8. • Remind students that, while strong acids and a vast majority of weak acids and weak bases are molecular substances, strong bases are ionic compounds. Lecture Outline1 16.1 Acids and Bases: A Brief Review2,3 • Acids taste sour and cause certain dyes to change color. -

Acid-Base Equilibria (Chapter 10.) Problems: 2,3,6,13,16,18,21,30,31,33
Chemistry 222 Acids and Bases Acid-Base Equilibria (Chapter 10.) Problems: 2,3,6,13,16,18,21,30,31,33 Review acid-base theory and titrations. For all titrations, at the equivalence point, the two reactants have completely reacted with one another according to the stoichiometry of the equation. For acids and bases with a 1:1 mole ratio, at the equivalence point of a titration, moles acid = moles base How do we find moles for a solutions? For a solid? Acids and bases are classified as strong (ionize completely) or weak (ionize only slightly.) An acid is a substance that can transfer a proton to another substance. A base is a substance that can accept a proton. A proton is a hydrogen ion, H+. Proton transfer is hydrogen ion transfer. + - Acid-base reactions: HCl + NH3 → NH4 + Cl *In every acid-base reaction, equilibrium favors transfer of a proton from the stronger acid to the stronger base. Water auto-ionizes to give equal, small concentrations of H+ and OH-. + - H2O(l) ⇔ H (aq) + OH (aq) + - 2 H2O(l) ⇔ H3O (aq) + OH (aq) + - Acid ionization in water: strong acid: HCl(aq) + H2O(l) → H3O (aq) + Cl (aq) Weak acid: HF(aq) + H2O(l) ⇔ How to write an equilibrium expression: What does the value of the equilibrium constant tell us about the reaction? + - Write the equilibrium expression and constant for water: 2 H2O(l) ⇔ H3O (aq) + OH (aq) Kw = Possible solutions: neutral acidic basic − In terms of [H+] and [OH ] The pH scale is a way to measure hydronium ion concentration: pH = pOH = The pH is a measure of acidity. -

Acidity of Hydrated Metal Ions Solution
Hydrated metal ions Charged metals ions also produce an acidic Acidity of hydrated metal ions solution. The metal itself does not act as a Brønsted-Lowry M = metal acid, but instead forms a hydrate that acts as a For Mn+, a small, highly charged ion, Brønsted-Lowry acid. n+ (n-1) + Salts and Molecular Structure M(H2O)x + H2O ⇌ M(H2O)x-1OH + H3O Typically the higher the charge on the metal ion, stronger the acidity of the hydrated ion. Calculate the pH of a 0.010 M AlCl3 solution. The Ka Cu2+ + 5 H O Cu(H O) 2+ 3+ -5 2 2 5 value for Al(H2O)6 is 1.4 x 10 . 2+ 2+ + Cu(H2O)5 CuOH(H2O)4 + H Salts of weak acids and bases Problem Molecular Trends in Acid For salts from a weak acid and weak base, the Predict whether an aqueous solutions of each of Ka and Kb of the ions must be compared. the following salts will be acidic, basic, or Strength neutral. If Ka > Kb the solution will be acidic K Al(H O) 3+ =1.4 x 10-5 NH C H O a 2 6 If Ka < Kb the solution will be basic 4 2 3 2 -4 Ka HF = 7.2 x 10 If K = K the solution will be neutral NH4CN - -2 a b Ka HSO4 = 1.2x10 Al2(SO4)3 - -10 Kb C2H3O2 = 5.6x10 NH F - -5 4 Kb CN = 1.6x10 -5 Kb NH3 = 1.8 x 10 Trends in Binary Nonmetal Oxyacids Hydride Activity Halogen Strong Acids With the same number of oxygen atoms This applies to nonmetals only All halogens are strong acids except fluorine. -

Acid-Base Strength Chapter 6
Acid-Base Strength Chapter 6 Monday, November 2, 2015 Acid-Base Strength We’ve seen that the reactivity of acids and bases can be viewed through the HSAB Model or the EC Model. • Both of these models try to provide a conceptual framework to explain empirical observations about acid-base reactivity • To determine the actual strength of an acid or of a base, different measurements can be made: • calorimetric measurements of reaction enthalpies (direct) • temperature dependence of Keq (direct) • spectroscopic measurements (IR, NMR, UV-Vis) (indirect) Direct Thermodynamic Measurements For reactions that go to completion, the enthalpy of the reaction can be determined directly from calorimetry HNO3 H 2O H 3O NO3 At constant pressure: qP E PV H internal ∆V ≅ 0 for enthalpy energy liquid phase Constant Pressure Calorimeter Direct Thermodynamic Measurements If a reaction doesn’t go to completion, the procedure is a little more complicated: We can use Hess’ Law and combine several different thermodynamic measurements to get the desired value: H1 CH 3COOH OH CH 3COO H 2O H2 H 3O OH 2H 2O Direct Thermodynamic Measurements If a reaction doesn’t go to completion, the procedure is a little more complicated: We can use Hess’ Law and combine several different thermodynamic measurements to get the desired value: H1 CH 3COOH OH CH 3COO H 2O H2 2H 2O H 3O OH Htotal H1 H 2 Direct Thermodynamic Measurements If a reaction doesn’t go to completion, the procedure is a little more complicated: Another alternative is to measure the temperature