Green Algae · Chlorophyta
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Early Photosynthetic Eukaryotes Inhabited Low-Salinity Habitats
Early photosynthetic eukaryotes inhabited PNAS PLUS low-salinity habitats Patricia Sánchez-Baracaldoa,1, John A. Ravenb,c, Davide Pisanid,e, and Andrew H. Knollf aSchool of Geographical Sciences, University of Bristol, Bristol BS8 1SS, United Kingdom; bDivision of Plant Science, University of Dundee at the James Hutton Institute, Dundee DD2 5DA, United Kingdom; cPlant Functional Biology and Climate Change Cluster, University of Technology Sydney, Ultimo, NSW 2007, Australia; dSchool of Biological Sciences, University of Bristol, Bristol BS8 1TH, United Kingdom; eSchool of Earth Sciences, University of Bristol, Bristol BS8 1TH, United Kingdom; and fDepartment of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02138 Edited by Peter R. Crane, Oak Spring Garden Foundation, Upperville, Virginia, and approved July 7, 2017 (received for review December 7, 2016) The early evolutionary history of the chloroplast lineage remains estimates for the origin of plastids ranging over 800 My (7). At the an open question. It is widely accepted that the endosymbiosis that same time, the ecological setting in which this endosymbiotic event established the chloroplast lineage in eukaryotes can be traced occurred has not been fully explored (8), partly because of phy- back to a single event, in which a cyanobacterium was incorpo- logenetic uncertainties and preservational biases of the fossil re- rated into a protistan host. It is still unclear, however, which cord. Phylogenomics and trait evolution analysis have pointed to a Cyanobacteria are most closely related to the chloroplast, when the freshwater origin for Cyanobacteria (9–11), providing an approach plastid lineage first evolved, and in what habitats this endosym- to address the early diversification of terrestrial biota for which the biotic event occurred. -

Phylogenetic Analysis of Rhizoclonium (Cladophoraceae, Cladophorales), and the Description of Rhizoclonium Subtile Sp
Phytotaxa 383 (2): 147–164 ISSN 1179-3155 (print edition) http://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2018 Magnolia Press Article ISSN 1179-3163 (online edition) https://doi.org/10.11646/phytotaxa.383.2.2 Phylogenetic analysis of Rhizoclonium (Cladophoraceae, Cladophorales), and the description of Rhizoclonium subtile sp. nov. from China ZHI-JUAN ZHAO1,2, HUAN ZHU3, GUO-XIANG LIU3* & ZHENG-YU HU4 1Key Laboratory of Environment Change and Resources Use in Beibu Gulf (Guangxi Teachers Education University), Ministry of Education, Nanning, 530001, P. R. China 2 Guangxi Key Laboratory of Earth Surface Processes and Intelligent Simulation (Guangxi Teachers Education University), Nanning, 530001, P. R. China 3Key Laboratory of Algal Biology, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, P. R. China 4State Key Laboratory of Freshwater Ecology and Biotechnology, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, P. R. China *e-mail:[email protected] Abstract The genus Rhizoclonium (Cladophoraceae, Cladophorales) accommodates uniserial, unbranched filamentous algae, closely related to Cladophora and Chaetomorpha. Its taxonomy has been problematic for a long time due to the lack of diagnostic morphological characters. To clarify the species diversity and taxonomic relationships of this genus, we collected and analyzed thirteen freshwater Rhizoclonium specimens from China. The morphological traits of these specimens were observed and described in detail. Three nuclear gene markers small subunit ribosomal DNA (SSU), large subunit ribosomal DNA (LSU) and internal transcribed spacer 2 (ITS2) sequences were analyzed to elucidate their phylogenetic relationships. The results revealed that there were at least fifteen molecular species assignable to Rhizoclonium and our thirteen specimens were distributed in four clades. -

Algae & Marine Plants of Point Reyes
Algae & Marine Plants of Point Reyes Green Algae or Chlorophyta Genus/Species Common Name Acrosiphonia coalita Green rope, Tangled weed Blidingia minima Blidingia minima var. vexata Dwarf sea hair Bryopsis corticulans Cladophora columbiana Green tuft alga Codium fragile subsp. californicum Sea staghorn Codium setchellii Smooth spongy cushion, Green spongy cushion Trentepohlia aurea Ulva californica Ulva fenestrata Sea lettuce Ulva intestinalis Sea hair, Sea lettuce, Gutweed, Grass kelp Ulva linza Ulva taeniata Urospora sp. Brown Algae or Ochrophyta Genus/Species Common Name Alaria marginata Ribbon kelp, Winged kelp Analipus japonicus Fir branch seaweed, Sea fir Coilodesme californica Dactylosiphon bullosus Desmarestia herbacea Desmarestia latifrons Egregia menziesii Feather boa Fucus distichus Bladderwrack, Rockweed Haplogloia andersonii Anderson's gooey brown Laminaria setchellii Southern stiff-stiped kelp Laminaria sinclairii Leathesia marina Sea cauliflower Melanosiphon intestinalis Twisted sea tubes Nereocystis luetkeana Bull kelp, Bullwhip kelp, Bladder wrack, Edible kelp, Ribbon kelp Pelvetiopsis limitata Petalonia fascia False kelp Petrospongium rugosum Phaeostrophion irregulare Sand-scoured false kelp Pterygophora californica Woody-stemmed kelp, Stalked kelp, Walking kelp Ralfsia sp. Silvetia compressa Rockweed Stephanocystis osmundacea Page 1 of 4 Red Algae or Rhodophyta Genus/Species Common Name Ahnfeltia fastigiata Bushy Ahnfelt's seaweed Ahnfeltiopsis linearis Anisocladella pacifica Bangia sp. Bossiella dichotoma Bossiella -

Morphometric Analysis of Surface Utricles in Halimeda Tuna (Bryopsidales, Ulvophyceae) Reveals Variation in Their Size and Symmetry Within Individual Segments
S S symmetry Article Morphometric Analysis of Surface Utricles in Halimeda tuna (Bryopsidales, Ulvophyceae) Reveals Variation in Their Size and Symmetry within Individual Segments Jiri Neustupa * and Yvonne Nemcova Department of Botany, Faculty of Science, Charles University, Prague, 12801 Benatska 2, Czech Republic; [email protected] * Correspondence: [email protected] Received: 26 June 2020; Accepted: 20 July 2020; Published: 1 August 2020 Abstract: Calcifying marine green algae of genus Halimeda have siphonous thalli composed of repeated segments. Their outer surface is formed by laterally appressed peripheral utricles which often form a honeycomb structure, typically with varying degrees of asymmetry in the individual polygons. This study is focused on a morphometric analysis of the size and symmetry of these polygons in Mediterranean H. tuna. Asymmetry of surface utricles is studied using a continuous symmetry measure quantifying the deviation of polygons from perfect symmetry. In addition, the segment shapes are also captured by geometric morphometrics and compared to the utricle parameters. The area of surface utricles is proved to be strongly related to their position on segments, where utricles near the segment bases are considerably smaller than those located near the apical and lateral margins. Interestingly, this gradient is most pronounced in relatively large reniform segments. The polygons are most symmetric in the central parts of segments, with asymmetry uniformly increasing towards the segment margins. Mean utricle asymmetry is found to be unrelated to segment shapes. Systematic differences in utricle size across different positions might be related to morphogenetic patterns of segment development, and may also indicate possible small-scale variations in CaCO3 content within segments. -

Internal Microbial Zonation Assists in the Massive Growth of Marimo, a Lake Ball of Aegagropila Linnaei in Lake Akan
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.20.434239; this version posted March 20, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Internal microbial zonation assists in the massive growth of marimo, a lake ball of 2 Aegagropila linnaei in Lake Akan 3 Ryosuke Nakai,1,* Isamu Wakana,2 Hironori Niki1,3,¶ 4 1 Microbial Physiology Laboratory, Department of Gene Function and Phenomics, National 5 Institute of Genetics, 1111, Yata, Mishima, Shizuoka 411-8540 Japan 6 2 Kushiro International Wetland Center, 7-5 Kuroganecho, Kushiro, Hokkaido 085-8505, Japan 7 3 Department of Genetics, SOKENDAI (The Graduate University for Advanced Studies), 1111, 8 Yata, Mishima, Shizuoka 411-8540 Japan 9 * Present address: Microbial Ecology and Technology Research Group, Bioproduction Research 10 Institute, National Institute of Advanced Industrial Science and Technology (AIST), 2-17-2-1, 11 Tsukisamu-higashi, Toyohira-ku, Sapporo, Hokkaido 062-8517 Japan 12 ¶ Correspondence to: Hironori Niki, Microbial Physiology Laboratory, Department of Gene 13 Function and Phenomics, National Institute of Genetics, 1111, Yata, Mishima, Shizuoka 411- 14 8540 Japan; E-mail: [email protected]; Tel: +81-55-981-6827; Fax: +81-55-981-6870 bioRxiv preprint doi: https://doi.org/10.1101/2021.03.20.434239; this version posted March 20, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 15 Abstract 16 Marimo (lake ball) is an uncommon ball-like aggregation of the green alga, Aegagropila linnaei. -

Neoproterozoic Origin and Multiple Transitions to Macroscopic Growth in Green Seaweeds
Neoproterozoic origin and multiple transitions to macroscopic growth in green seaweeds Andrea Del Cortonaa,b,c,d,1, Christopher J. Jacksone, François Bucchinib,c, Michiel Van Belb,c, Sofie D’hondta, f g h i,j,k e Pavel Skaloud , Charles F. Delwiche , Andrew H. Knoll , John A. Raven , Heroen Verbruggen , Klaas Vandepoeleb,c,d,1,2, Olivier De Clercka,1,2, and Frederik Leliaerta,l,1,2 aDepartment of Biology, Phycology Research Group, Ghent University, 9000 Ghent, Belgium; bDepartment of Plant Biotechnology and Bioinformatics, Ghent University, 9052 Zwijnaarde, Belgium; cVlaams Instituut voor Biotechnologie Center for Plant Systems Biology, 9052 Zwijnaarde, Belgium; dBioinformatics Institute Ghent, Ghent University, 9052 Zwijnaarde, Belgium; eSchool of Biosciences, University of Melbourne, Melbourne, VIC 3010, Australia; fDepartment of Botany, Faculty of Science, Charles University, CZ-12800 Prague 2, Czech Republic; gDepartment of Cell Biology and Molecular Genetics, University of Maryland, College Park, MD 20742; hDepartment of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02138; iDivision of Plant Sciences, University of Dundee at the James Hutton Institute, Dundee DD2 5DA, United Kingdom; jSchool of Biological Sciences, University of Western Australia, WA 6009, Australia; kClimate Change Cluster, University of Technology, Ultimo, NSW 2006, Australia; and lMeise Botanic Garden, 1860 Meise, Belgium Edited by Pamela S. Soltis, University of Florida, Gainesville, FL, and approved December 13, 2019 (received for review June 11, 2019) The Neoproterozoic Era records the transition from a largely clear interpretation of how many times and when green seaweeds bacterial to a predominantly eukaryotic phototrophic world, creat- emerged from unicellular ancestors (8). ing the foundation for the complex benthic ecosystems that have There is general consensus that an early split in the evolution sustained Metazoa from the Ediacaran Period onward. -

The Moss-Back Alga (Cladophorophyceae, Chlorophyta) on Two Species of Freshwater Turtles in the Kimberleys
Telopea 12(2) 279–284 The moss-back alga (Cladophorophyceae, Chlorophyta) on two species of freshwater turtles in the Kimberleys Stephen Skinner1,2, Nancy FitzSimmons3 and Timothy J. Entwisle1 1National Herbarium of New South Wales, Mrs Macquaries Road, Sydney NSW 2000 Australia 2Southern ACT Catchment Group Inc., PO Box 2056, Kambah, ACT Author for correspondence: [email protected] 3Institute for Applied Ecology, School of Resource, Environmental & Heritage Sciences, University of Canberra, Canberra, ACT 2601, Australia Abstract The range of the Australian freshwater alga Basicladia ramulosa Ducker is extended, both in its turtle hosts (Chelodina burrungandjii Thomson et al.; Emydura australis (Grey)) and in geography, to tropical northern Western Australia. Along with further morphological observations, sporangia are described for the first time in this taxon. Introduction Moss-back turtles (Fig. 1) have fascinated biologists for many years. While the carapace of a potentially amphibious turtle would be a challenging habitat for most aquatic organisms, it is perhaps surprising there are only a handful of attached algae reported from such sites. Edgren et al. (1953) detailed the range of host turtles then known in North America and the range of epizoic algae that included Rhizoclonium and Cladophora. Two further genera in the Cladophoraceae are the only macroalgae widely reported on turtle carapaces: the prostrate, spreading, endozoic (and possibly disease causing) Dermatophyton radicans Peter, and species of the heterotrichous genus Basicladia, responsible for the name ‘moss-back’. In the United States, Basicladia is considered a small epizoic genus on turtles and water snails, of three to four taxa (John 2003). Hamilton (1948) described sexual reproduction in North American species of Basicladia involving the fusion of biflagellate zooids as is commonly the case in the Cladophoraceae. -

1 Integrative Biology 200 "PRINCIPLES OF
Integrative Biology 200 "PRINCIPLES OF PHYLOGENETICS" Spring 2018 University of California, Berkeley B.D. Mishler March 14, 2018. Classification II: Phylogenetic taxonomy including incorporation of fossils; PhyloCode I. Phylogenetic Taxonomy - the argument for rank-free classification A number of recent calls have been made for the reformation of the Linnaean hierarchy (e.g., De Queiroz & Gauthier, 1992). These authors have emphasized that the existing system is based in a non-evolutionary world-view; the roots of the Linnaean hierarchy are in a specially- created world-view. Perhaps the idea of fixed, comparable ranks made some sense under that view, but under an evolutionary world view they don't make sense. There are several problems with the current nomenclatorial system: 1. The current system, with its single type for a name, cannot be used to precisely name a clade. E.g., you may name a family based on a certain type specimen, and even if you were clear about what node you meant to name in your original publication, the exact phylogenetic application of your name would not be clear subsequently, after new clades are added. 2. There are not nearly enough ranks to name the thousands of levels of monophyletic groups in the tree of life. Therefore people are increasingly using informal rank-free names for higher- level nodes, but without any clear, formal specification of what clade is meant. 3. Most aspects of the current code, including priority, revolve around the ranks, which leads to instability of usage. For example, when a change in relationships is discovered, several names often need to be changed to adjust, including those of groups whose circumscription has not changed. -

Langston R and H Spalding. 2017
A survey of fishes associated with Hawaiian deep-water Halimeda kanaloana (Bryopsidales: Halimedaceae) and Avrainvillea sp. (Bryopsidales: Udoteaceae) meadows Ross C. Langston1 and Heather L. Spalding2 1 Department of Natural Sciences, University of Hawai`i- Windward Community College, Kane`ohe,¯ HI, USA 2 Department of Botany, University of Hawai`i at Manoa,¯ Honolulu, HI, USA ABSTRACT The invasive macroalgal species Avrainvillea sp. and native species Halimeda kanaloana form expansive meadows that extend to depths of 80 m or more in the waters off of O`ahu and Maui, respectively. Despite their wide depth distribution, comparatively little is known about the biota associated with these macroalgal species. Our primary goals were to provide baseline information on the fish fauna associated with these deep-water macroalgal meadows and to compare the abundance and diversity of fishes between the meadow interior and sandy perimeters. Because both species form structurally complex three-dimensional canopies, we hypothesized that they would support a greater abundance and diversity of fishes when compared to surrounding sandy areas. We surveyed the fish fauna associated with these meadows using visual surveys and collections made with clove-oil anesthetic. Using these techniques, we recorded a total of 49 species from 25 families for H. kanaloana meadows and surrounding sandy areas, and 28 species from 19 families for Avrainvillea sp. habitats. Percent endemism was 28.6% and 10.7%, respectively. Wrasses (Family Labridae) were the most speciose taxon in both habitats (11 and six species, respectively), followed by gobies for H. kanaloana (six Submitted 18 November 2016 species). The wrasse Oxycheilinus bimaculatus and cardinalfish Apogonichthys perdix Accepted 13 April 2017 were the most frequently-occurring species within the H. -
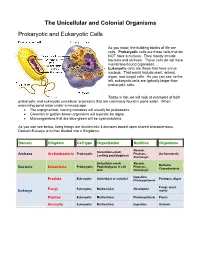
The Unicellular and Colonial Organisms Prokaryotic And
The Unicellular and Colonial Organisms Prokaryotic and Eukaryotic Cells As you know, the building blocks of life are cells. Prokaryotic cells are those cells that do NOT have a nucleus. They mostly include bacteria and archaea. These cells do not have membrane-bound organelles. Eukaryotic cells are those that have a true nucleus. That would include plant, animal, algae, and fungal cells. As you can see, to the left, eukaryotic cells are typically larger than prokaryotic cells. Today in lab, we will look at examples of both prokaryotic and eukaryotic unicellular organisms that are commonly found in pond water. When examining pond water under a microscope… The unpigmented, moving microbes will usually be protozoans. Greenish or golden-brown organisms will typically be algae. Microorganisms that are blue-green will be cyanobacteria. As you can see below, living things are divided into 3 domains based upon shared characteristics. Domain Eukarya is further divided into 4 Kingdoms. Domain Kingdom Cell type Organization Nutrition Organisms Absorb, Unicellular-small; Prokaryotic Photsyn., Archaeacteria Archaea Archaebacteria Lacking peptidoglycan Chemosyn. Unicellular-small; Absorb, Bacteria, Prokaryotic Peptidoglycan in cell Photsyn., Bacteria Eubacteria Cyanobacteria wall Chemosyn. Ingestion, Eukaryotic Unicellular or colonial Protozoa, Algae Protista Photosynthesis Fungi, yeast, Fungi Eukaryotic Multicellular Absorption Eukarya molds Plantae Eukaryotic Multicellular Photosynthesis Plants Animalia Eukaryotic Multicellular Ingestion Animals Prokaryotic Organisms – the archaea, non-photosynthetic bacteria, and cyanobacteria Archaea - Microorganisms that resemble bacteria, but are different from them in certain aspects. Archaea cell walls do not include the macromolecule peptidoglycan, which is always found in the cell walls of bacteria. Archaea usually live in extreme, often very hot or salty environments, such as hot mineral springs or deep-sea hydrothermal vents. -

Plate. Acetabularia Schenckii
Training in Tropical Taxonomy 9-23 July, 2008 Tropical Field Phycology Workshop Field Guide to Common Marine Algae of the Bocas del Toro Area Margarita Rosa Albis Salas David Wilson Freshwater Jesse Alden Anna Fricke Olga Maria Camacho Hadad Kevin Miklasz Rachel Collin Andrea Eugenia Planas Orellana Martha Cecilia Díaz Ruiz Jimena Samper Villareal Amy Driskell Liz Sargent Cindy Fernández García Thomas Sauvage Ryan Fikes Samantha Schmitt Suzanne Fredericq Brian Wysor From July 9th-23rd, 2008, 11 graduate and 2 undergraduate students representing 6 countries (Colombia, Costa Rica, El Salvador, Germany, France and the US) participated in a 15-day Marine Science Network-sponsored workshop on Tropical Field Phycology. The students and instructors (Drs. Brian Wysor, Roger Williams University; Wilson Freshwater, University of North Carolina at Wilmington; Suzanne Fredericq, University of Louisiana at Lafayette) worked synergistically with the Smithsonian Institution's DNA Barcode initiative. As part of the Bocas Research Station's Training in Tropical Taxonomy program, lecture material included discussions of the current taxonomy of marine macroalgae; an overview and recent assessment of the diagnostic vegetative and reproductive morphological characters that differentiate orders, families, genera and species; and applications of molecular tools to pertinent questions in systematics. Instructors and students collected multiple samples of over 200 algal species by SCUBA diving, snorkeling and intertidal surveys. As part of the training in tropical taxonomy, many of these samples were used by the students to create a guide to the common seaweeds of the Bocas del Toro region. Herbarium specimens will be contributed to the Bocas station's reference collection and the University of Panama Herbarium. -

The Marine Species of Cladophora (Chlorophyta) from the South African East Coast
NovaHedwigia 76 1—2 45—82 Stuttgart, Februar 2003 The marine species of Cladophora (Chlorophyta) from the South African East Coast by F. Leliaert and E. Coppejans Research Group Phycology, Department of Biology, Ghent University, Krijgslaan 281, S8 B-9000 Ghent, Belgium E-mails: [email protected] and [email protected] With 16 figures and 5 tables Leliaert, F. & E. Coppejans (2003): The marine species of Cladophora (Chlorophyta) from the South African East Coast. - Nova Hedwigia 76: 45-82. Abstract: Twelve species of the genus Cladophora occur along the South African East Coast. Detailed descriptions and illustrations are presented. Four species are recorded for the first time in South Africa: C. catenata , C. vagabunda , C. horii and C. dotyana; the last two are also new records for the Indian Ocean. A comparison of the South African C. rugulosa specimens with specimens of C. prolifera from South Africa and other regions have shown that these species are not synonymous as previously considered, leading to the resurrection of C. rugulosa which is probably a South African endemic. Key words: Cladophora, C. catenata , C. dotyana, C. horii, C. prolifera , C. rugulosa , C. vagabunda , South Africa, KwaZulu-Natal. Introduction Cladophora Kützing is one of the largest green-algal genera and has a worldwide distribution. Within the class Cladophorophyceae the genus Cladophora is characterized by its simple thallus architecture: branched, uniseriate filaments of multinucleate cells. Eleven different architectural types (sections) are distinguished in the genus (van den Hoek 1963, 1982; van den Hoek & Chihara 2000). Recent studies based on morphological and molecular data have proven that Cladophora is polyphyletic (van den Hoek 1982; Bakker et al.