Monoamine Oxidase Inhibition by Kavalactones from Kava (Piper Methysticum)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
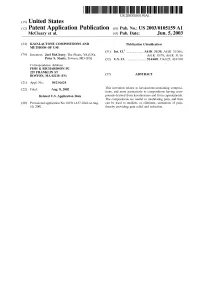
(12) Patent Application Publication (10) Pub. No.: US 2003/0105159 A1 Mccleary Et Al
US 200301 05159A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0105159 A1 McCleary et al. (43) Pub. Date: Jun. 5, 2003 (54) KAVALACTONE COMPOSITIONS AND Publication Classification METHODS OF USE (51) Int. Cl." ....................... A61K 31/35; A61K 31/366; (76) Inventors: Joel McCleary, The Plains, VA (US); A61K 35/78; A61K 31/16 Peter S. Staats, Towson, MD (US) (52) U.S. Cl. ........................... 514/460; 514/625; 424/760 Correspondence Address: FISH & RICHARDSON PC 225 FRANKLIN ST BOSTON, MA 02110 (US) (57) ABSTRACT (21) Appl. No.: 10/214,624 (22) Filed: Aug. 8, 2002 This invention relates tO kavalactone-containing composi tions, and more particularly to compositions having com Related U.S. Application Data pounds derived from kavalactones and from capsaicinoids. The compositions are useful in modulating pain, and thus (60) Provisional application No. 60/311,437, filed on Aug. can be used to mediate, or eliminate, Sensations of pain, 10, 2001. thereby providing pain relief and reduction. US 2003/0105159 A1 Jun. 5, 2003 KAVALACTONE COMPOSITIONS AND METHODS 0006. In one embodiment, the invention relates to an OF USE analgesic topical composition having: (a) a kavalactone; (b) capsaicinoid or Synthetic derivatives thereof; and (c) a CROSS-REFERENCE TO RELATED pharmaceutically acceptable carrier; wherein the weight APPLICATIONS ratio of(a):(b) is from 5000:1 to 1:2 (e.g., 800:1 to 1:1; 500:1 to 5:1). In other aspects, the composition includes an effec 0001. This application claims benefit of U.S. application tive amount of kavalactones, active kavalactones, or capsai Ser. -

Summary of Product Characteristics
Package leaflet: Information for the patient Fluoxetine 20mg Capsules, hard fluoxetine Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. • Keep this leaflet. You may need to read it again. • If you have further questions, ask your doctor or pharmacist. • This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. •If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. What is in this leaflet 1. What Fluoxetine is and what it is used for 2. What you need to know before you take Fluoxetine 3. How to take Fluoxetine 4. Possible side effects 5. How to store Fluoxetine 6. Contents of the pack and other information 1. What Fluoxetine is and what it is used for The name of your medicine is Fluoxetine 20mg Capsules, hard. It contains the active substance fluoxetine. Fluoxetine belongs to a group of medicines called selective serotonin reuptake inhibitor (SSRI) antidepressants. Fluoxetine can be given to treat the following conditions: Adults: • Major depressive episodes • The symptoms of a condition called obsessive-compulsive disorder (OCD). • The eating disorder bulimia nervosa. This medicine is used alongside psychotherapy for the reduction of binge-eating and purging. Children and adolescents aged 8 years and above: • Moderate to severe major depressive disorder, if the depression does not respond to psychological therapy after 4-6 sessions. Fluoxetine should be offered to a child or young person with moderate to severe major depressive disorder only in combination with psychological therapy. -

Novel Neuroprotective Compunds for Use in Parkinson's Disease
Novel neuroprotective compounds for use in Parkinson’s disease A thesis submitted to Kent State University in partial Fulfillment of the requirements for the Degree of Master of Science By Ahmed Shubbar December, 2013 Thesis written by Ahmed Shubbar B.S., University of Kufa, 2009 M.S., Kent State University, 2013 Approved by ______________________Werner Geldenhuys ____, Chair, Master’s Thesis Committee __________________________,Altaf Darvesh Member, Master’s Thesis Committee __________________________,Richard Carroll Member, Master’s Thesis Committee ___Eric_______________________ Mintz , Director, School of Biomedical Sciences ___Janis_______________________ Crowther , Dean, College of Arts and Sciences ii Table of Contents List of figures…………………………………………………………………………………..v List of tables……………………………………………………………………………………vi Acknowledgments.…………………………………………………………………………….vii Chapter 1: Introduction ..................................................................................... 1 1.1 Parkinson’s disease .............................................................................................. 1 1.2 Monoamine Oxidases ........................................................................................... 3 1.3 Monoamine Oxidase-B structure ........................................................................... 8 1.4 Structural differences between MAO-B and MAO-A .............................................13 1.5 Mechanism of oxidative deamination catalyzed by Monoamine Oxidases ............15 1 .6 Neuroprotective effects -

(12) Patent Application Publication (10) Pub. No.: US 2016/017.4603 A1 Abayarathna Et Al
US 2016O174603A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/017.4603 A1 Abayarathna et al. (43) Pub. Date: Jun. 23, 2016 (54) ELECTRONIC VAPORLIQUID (52) U.S. Cl. COMPOSITION AND METHOD OF USE CPC ................. A24B 15/16 (2013.01); A24B 15/18 (2013.01); A24F 47/002 (2013.01) (71) Applicants: Sahan Abayarathna, Missouri City, TX 57 ABSTRACT (US); Michael Jaehne, Missouri CIty, An(57) e-liquid for use in electronic cigarettes which utilizes- a TX (US) vaporizing base (either propylene glycol, vegetable glycerin, (72) Inventors: Sahan Abayarathna, MissOU1 City,- 0 TX generallyor mixture at of a 0.001 the two) g-2.0 mixed g per with 1 mL an ratio. herbal The powder herbal extract TX(US); (US) Michael Jaehne, Missouri CIty, can be any of the following:- - - Kanna (Sceletium tortuosum), Blue lotus (Nymphaea caerulea), Salvia (Salvia divinorum), Salvia eivinorm, Kratom (Mitragyna speciosa), Celandine (21) Appl. No.: 14/581,179 poppy (Stylophorum diphyllum), Mugwort (Artemisia), Coltsfoot leaf (Tussilago farfara), California poppy (Eschscholzia Californica), Sinicuichi (Heimia Salicifolia), (22) Filed: Dec. 23, 2014 St. John's Wort (Hypericum perforatum), Yerba lenna yesca A rtemisia scoparia), CaleaCal Zacatechichihichi (Calea(Cal termifolia), Leonurus Sibericus (Leonurus Sibiricus), Wild dagga (Leono Publication Classification tis leonurus), Klip dagga (Leonotis nepetifolia), Damiana (Turnera diffiisa), Kava (Piper methysticum), Scotch broom (51) Int. Cl. tops (Cytisus scoparius), Valarien (Valeriana officinalis), A24B 15/16 (2006.01) Indian warrior (Pedicularis densiflora), Wild lettuce (Lactuca A24F 47/00 (2006.01) virosa), Skullcap (Scutellaria lateriflora), Red Clover (Trifo A24B I5/8 (2006.01) lium pretense), and/or combinations therein. -

The Self-Inhibitory Nature of Metabolic Networks and Its Alleviation Through Compartmentalization
ARTICLE Received 30 Oct 2016 | Accepted 23 May 2017 | Published 10 Jul 2017 DOI: 10.1038/ncomms16018 OPEN The self-inhibitory nature of metabolic networks and its alleviation through compartmentalization Mohammad Tauqeer Alam1,2, Viridiana Olin-Sandoval1,3, Anna Stincone1,w, Markus A. Keller1,4, Aleksej Zelezniak1,5,6, Ben F. Luisi1 & Markus Ralser1,5 Metabolites can inhibit the enzymes that generate them. To explore the general nature of metabolic self-inhibition, we surveyed enzymological data accrued from a century of experimentation and generated a genome-scale enzyme-inhibition network. Enzyme inhibition is often driven by essential metabolites, affects the majority of biochemical processes, and is executed by a structured network whose topological organization is reflecting chemical similarities that exist between metabolites. Most inhibitory interactions are competitive, emerge in the close neighbourhood of the inhibited enzymes, and result from structural similarities between substrate and inhibitors. Structural constraints also explain one-third of allosteric inhibitors, a finding rationalized by crystallographic analysis of allosterically inhibited L-lactate dehydrogenase. Our findings suggest that the primary cause of metabolic enzyme inhibition is not the evolution of regulatory metabolite–enzyme interactions, but a finite structural diversity prevalent within the metabolome. In eukaryotes, compartmentalization minimizes inevitable enzyme inhibition and alleviates constraints that self-inhibition places on metabolism. 1 Department of Biochemistry and Cambridge Systems Biology Centre, University of Cambridge, 80 Tennis Court Road, Cambridge CB2 1GA, UK. 2 Division of Biomedical Sciences, Warwick Medical School, University of Warwick, Gibbet Hill Road, Coventry CV4 7AL, UK. 3 Department of Food Science and Technology, Instituto Nacional de Ciencias Me´dicas y Nutricio´n Salvador Zubira´n, Vasco de Quiroga 15, Tlalpan, 14080 Mexico City, Mexico. -

Yangonin Blocks Tumor Necrosis Factor-Α–Induced Nuclear Factor-Κb–Dependent Transcription by Inhibiting the Transactivation Potential of the Rela/P65 Subunit
J Pharmacol Sci 118, 447 – 454 (2012) Journal of Pharmacological Sciences © The Japanese Pharmacological Society Full Paper Yangonin Blocks Tumor Necrosis Factor-α–Induced Nuclear Factor-κB–Dependent Transcription by Inhibiting the Transactivation Potential of the RelA/p65 Subunit Juan Ma1,†, He Liang1,†, Hong Ri Jin2, Nguyen Tien Dat3, Shan Yu Zhang1, Ying Zi Jiang1, Ji Xing Nan1, Donghao Li1, Xue Wu1, Jung Joon Lee1,2,*a, and Xuejun Jin1,*b 1Key Laboratory of Natural Resources of Changbai Mountain & Functional Molecules, Yanbian University, Ministry of Education, Yanji Jilin 133002, China 2Center for Molecular Cancer Research, Korea Research Institute of Bioscience and Biotechnology, Ochang, Chungbuk 363-883, Republic of Korea 3Institute of Marine Biochemistry, Vietnam Academy of Science and Technology, 18-Hoang Quoc Viet, Hanoi, Vietnam Received November 13, 2011; Accepted January 23, 2012 Abstract. The nuclear factor-κB (NF-κB) transcription factors control many physiological pro- cesses including inflammation, immunity, and apoptosis. In our search for NF-κB inhibitors from natural resources, we identified yangonin from Piper methysticum as an inhibitor of NF-κB activa- tion. In the present study, we demonstrate that yangonin potently inhibits NF-κB activation through suppression of the transcriptional activity of the RelA/p65 subunit of NF-κB. This compound sig- nificantly inhibited the induced expression of the NF-κB-reporter gene. However, this compound did not interfere with tumor necrosis factor-α (TNF-α)-induced inhibitor of κBα (IκBα) degrada- tion, p65 nuclear translocation, and DNA-binding activity of NF-κB. Further analysis revealed that yangonin inhibited not only the induced NF-κB activation by overexpression of RelA/p65, but also transactivation activity of RelA/p65. -

The In¯Uence of Medication on Erectile Function
International Journal of Impotence Research (1997) 9, 17±26 ß 1997 Stockton Press All rights reserved 0955-9930/97 $12.00 The in¯uence of medication on erectile function W Meinhardt1, RF Kropman2, P Vermeij3, AAB Lycklama aÁ Nijeholt4 and J Zwartendijk4 1Department of Urology, Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands; 2Department of Urology, Leyenburg Hospital, Leyweg 275, 2545 CH The Hague, The Netherlands; 3Pharmacy; and 4Department of Urology, Leiden University Hospital, P.O. Box 9600, 2300 RC Leiden, The Netherlands Keywords: impotence; side-effect; antipsychotic; antihypertensive; physiology; erectile function Introduction stopped their antihypertensive treatment over a ®ve year period, because of side-effects on sexual function.5 In the drug registration procedures sexual Several physiological mechanisms are involved in function is not a major issue. This means that erectile function. A negative in¯uence of prescrip- knowledge of the problem is mainly dependent on tion-drugs on these mechanisms will not always case reports and the lists from side effect registries.6±8 come to the attention of the clinician, whereas a Another way of looking at the problem is drug causing priapism will rarely escape the atten- combining available data on mechanisms of action tion. of drugs with the knowledge of the physiological When erectile function is in¯uenced in a negative mechanisms involved in erectile function. The way compensation may occur. For example, age- advantage of this approach is that remedies may related penile sensory disorders may be compen- evolve from it. sated for by extra stimulation.1 Diminished in¯ux of In this paper we will discuss the subject in the blood will lead to a slower onset of the erection, but following order: may be accepted. -

Acetylcholinesterase and Monoamine Oxidase-B Inhibitory Activities By
www.nature.com/scientificreports OPEN Acetylcholinesterase and monoamine oxidase‑B inhibitory activities by ellagic acid derivatives isolated from Castanopsis cuspidata var. sieboldii Jong Min Oh1, Hyun‑Jae Jang2, Myung‑Gyun Kang3, Soobin Song2, Doo‑Young Kim2, Jung‑Hee Kim2, Ji‑In Noh1, Jong Eun Park1, Daeui Park3, Sung‑Tae Yee1 & Hoon Kim1* Among 276 herbal extracts, a methanol extract of Castanopsis cuspidata var. sieboldii stems was selected as an experimental source for novel acetylcholinesterase (AChE) inhibitors. Five compounds were isolated from the extract by activity‑guided screening, and their inhibitory activities against butyrylcholinesterase (BChE), monoamine oxidases (MAOs), and β‑site amyloid precursor protein cleaving enzyme 1 (BACE‑1) were also evaluated. Of these compounds, 4′‑O‑(α‑l‑rhamnopyranosyl)‑ 3,3′,4‑tri‑O‑methylellagic acid (3) and 3,3′,4‑tri‑O‑methylellagic acid (4) efectively inhibited AChE with IC50 values of 10.1 and 10.7 µM, respectively. Ellagic acid (5) inhibited AChE (IC50 = 41.7 µM) less than 3 and 4. In addition, 3 efectively inhibited MAO‑B (IC50 = 7.27 µM) followed by 5 (IC50 = 9.21 µM). All fve compounds weakly inhibited BChE and BACE‑1. Compounds 3, 4, and 5 reversibly and competitively inhibited AChE, and were slightly or non‑toxic to MDCK cells. The binding energies of 3 and 4 (− 8.5 and − 9.2 kcal/mol, respectively) for AChE were greater than that of 5 (− 8.3 kcal/mol), and 3 and 4 formed a hydrogen bond with Tyr124 in AChE. These results suggest 3 is a dual‑targeting inhibitor of AChE and MAO‑B, and that these compounds should be viewed as potential therapeutics for the treatment of Alzheimer’s disease. -

Questions in the Chemical Enzymology of MAO
Review Questions in the Chemical Enzymology of MAO Rona R. Ramsay 1,* and Alen Albreht 2 1 Biomedical Sciences Research Complex, School of Biology, University of St Andrews, St Andrews KY16 9ST, UK 2 Laboratory for Food Chemistry, Department of Analytical Chemistry, National Institute of Chemistry, Hajdrihova 19, SI-1000 Ljubljana, Slovenia; [email protected] * Correspondence: [email protected]; Tel.: +44-(0)-1334-474740 Abstract: We have structure, a wealth of kinetic data, thousands of chemical ligands and clinical information for the effects of a range of drugs on monoamine oxidase activity in vivo. We have comparative information from various species and mutations on kinetics and effects of inhibition. Nevertheless, there are what seem like simple questions still to be answered. This article presents a brief summary of existing experimental evidence the background and poses questions that remain intriguing for chemists and biochemists researching the chemical enzymology of and drug design for monoamine oxidases (FAD-containing EC 4.1.3.4). Keywords: chemical mechanism; kinetic mechanism; oxidation; protein flexibility; cysteine modifica- tion; reversible/irreversible inhibition; molecular dynamics; simulation 1. Introduction Monoamine oxidase (E.C. 1.4.3.4) enzymes MAO A and MAO B are FAD-containing Citation: Ramsay, R.R.; Albreht, A. proteins located on the outer face of the mitochondrial inner membrane, retained there Questions in the Chemical Enzymology of MAO. Chemistry 2021, by hydrophobic interactions and a transmembrane helix. The redox co-factor (FAD) is 3, 959–978. https://doi.org/10.3390/ covalently attached to a cysteine and buried deep inside the protein [1]. -

Plant Protease Inhibitors: a Defense Strategy in Plants
Biotechnology and Molecular Biology Review Vol. 2 (3), pp. 068-085, August 2007 Available online at http://www.academicjournals.org/BMBR ISSN 1538-2273 © 2007 Academic Journals Standard Review Plant protease inhibitors: a defense strategy in plants Huma Habib and Khalid Majid Fazili* Department of Biotechnology, The University of Kashmir, P/O Naseembagh, Hazratbal, Srinagar -190006, Jammu and Kashmir, India. Accepted 7 July, 2007 Proteases, though essentially indispensable to the maintenance and survival of their host organisms, can be potentially damaging when overexpressed or present in higher concentrations, and their activities need to be correctly regulated. An important means of regulation involves modulation of their activities through interaction with substances, mostly proteins, called protease inhibitors. Some insects and many of the phytopathogenic microorganisms secrete extracellular enzymes and, in particular, enzymes causing proteolytic digestion of proteins, which play important roles in pathogenesis. Plants, however, have also developed mechanisms to fight these pathogenic organisms. One important line of defense that plants have to fight these pathogens is through various inhibitors that act against these proteolytic enzymes. These inhibitors are thus active in endogenous as well as exogenous defense systems. Protease inhibitors active against different mechanistic classes of proteases have been classified into different families on the basis of significant sequence similarities and structural relationships. Specific protease inhibitors are currently being overexpressed in certain transgenic plants to protect them against invaders. The current knowledge about plant protease inhibitors, their structure and their role in plant defense is briefly reviewed. Key words: Proteases, enzymes, protease inhibitors, serpins, cystatins, pathogens, defense. Table of content 1. -

Herbal Insomnia Medications That Target Gabaergic Systems: a Review of the Psychopharmacological Evidence
Send Orders for Reprints to [email protected] Current Neuropharmacology, 2014, 12, 000-000 1 Herbal Insomnia Medications that Target GABAergic Systems: A Review of the Psychopharmacological Evidence Yuan Shia, Jing-Wen Donga, Jiang-He Zhaob, Li-Na Tanga and Jian-Jun Zhanga,* aState Key Laboratory of Bioactive Substance and Function of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, P.R. China; bDepartment of Pharmacology, School of Marine, Shandong University, Weihai, P.R. China Abstract: Insomnia is a common sleep disorder which is prevalent in women and the elderly. Current insomnia drugs mainly target the -aminobutyric acid (GABA) receptor, melatonin receptor, histamine receptor, orexin, and serotonin receptor. GABAA receptor modulators are ordinarily used to manage insomnia, but they are known to affect sleep maintenance, including residual effects, tolerance, and dependence. In an effort to discover new drugs that relieve insomnia symptoms while avoiding side effects, numerous studies focusing on the neurotransmitter GABA and herbal medicines have been conducted. Traditional herbal medicines, such as Piper methysticum and the seed of Zizyphus jujuba Mill var. spinosa, have been widely reported to improve sleep and other mental disorders. These herbal medicines have been applied for many years in folk medicine, and extracts of these medicines have been used to study their pharmacological actions and mechanisms. Although effective and relatively safe, natural plant products have some side effects, such as hepatotoxicity and skin reactions effects of Piper methysticum. In addition, there are insufficient evidences to certify the safety of most traditional herbal medicine. In this review, we provide an overview of the current state of knowledge regarding a variety of natural plant products that are commonly used to treat insomnia to facilitate future studies. -

Effect of Statins and ACE Inhibitors Alone and in Combination on Clinical Outcome in Patients with Coronary Heart Disease
Journal of Human Hypertension (2004) 18, 781–788 & 2004 Nature Publishing Group All rights reserved 0950-9240/04 $30.00 www.nature.com/jhh ORIGINAL ARTICLE Effect of statins and ACE inhibitors alone and in combination on clinical outcome in patients with coronary heart disease VG Athyros1, DP Mikhailidis3, AA Papageorgiou2, VI Bouloukos1, AN Pehlivanidis1, AN Symeonidis4 and M Elisaf5, for the GREACE Study Collaborative Group 1Atherosclerosis Unit, Aristotelian University, Hippocration Hospital, Thessaloniki, Greece; 2Department of Clinical Biochemistry, Royal Free Hospital, Royal Free and University College Medical School, Pond Street, London, UK; 32nd Propedeutic Department of Internal Medicine, Aristotelian University, Hippocration Hospital, Thessaloniki, Greece; 4Greek Society of General Practitioners, Thessaloniki, Greece; 5Department of Internal Medicine, Medical School, University of Ioannina, Greece We assessed the ‘synergy’ of statins and angiotensin- point in group A was 31%, (95% CI À48 to À6%, P ¼ 0.01) converting enzyme inhibitors (ACEI) in reducing vascu- in comparison to group B, 59% (95% CI À72 to À48%, lar events in patients with coronary heart disease (CHD). Po0.0001) to group C and 63% (95% CI À74 to À51%, The GREek Atorvastatin and CHD Evaluation (GREACE) Po0.0001) to group D. There was no significant Study, suggested that aggressive reduction of low difference in RRR between groups C and D (9%, CI density lipoprotein cholesterol to 2.59 mmol/l À27–10%, P ¼ 0.1). Other factors (eg the blood pressure) (o100 mg/dl) significantly reduces morbidity and mor- that can influence clinical outcome did not differ tality in CHD patients, in comparison to undertreated significantly between the four treatment groups.