Gastroparesis Part 3, Dumping Syndrome
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reactive Hypoglycemia from Metformin Immediate-Release Monotherapy Resolved by a Switch to Metformin Extended-Release: Conceptualizing Their Concentration-Time Curves
Open Access Case Report DOI: 10.7759/cureus.16112 Reactive Hypoglycemia From Metformin Immediate-Release Monotherapy Resolved by a Switch to Metformin Extended-Release: Conceptualizing Their Concentration-Time Curves Ayesha Akram 1, 2 1. Internal Medicine, Combined Military Hospital, Rawalpindi, PAK 2. Internal Medicine, Rawalpindi Medical University, Rawalpindi, PAK Corresponding author: Ayesha Akram, [email protected] Abstract Metformin rarely, if ever, causes hypoglycemia when it is used as labeled. A 55-year-old woman presented to the medicine ward with an altered level of consciousness. She had been reviewed in an outpatient department three days earlier and prescribed 500 mg two times per day of metformin immediate-release (Met IR) for newly diagnosed type 2 diabetes mellitus (T2DM), to which she had been adherent; however, she had been experiencing intermittent episodes of hypoglycemia after taking the medication prescribed to treat her T2DM. On physical examination, she was diaphoretic and disoriented but responsive to sensory stimuli. In the ward, she received 25 ml of intravenous dextrose as the initial blood glucose reading was low at 54 mg/dl, and 4 ounces of apple juice additionally two hours later as her blood glucose level fell below 70 mg/dl again. She was no longer hypoglycemic a few hours later, and there was a significant neurological improvement. The remainder of the laboratory results, including serum renal and liver function tests, were normal. Met IR was discontinued, and metformin extended-release (Met XR) 500 mg/day was initiated at discharge. The patient's hypoglycemic episodes resolved within days after the initiation of Met XR. -

Total Pancreatectomy for Chronic Pancreatitis
Gut: first published as 10.1136/gut.29.3.358 on 1 March 1988. Downloaded from Gut, 1988, 29, 358-365 Total pancreatectomy for chronic pancreatitis I P LINEHAN, M A LAMBERT, D C BROWN, A B KURTZ, P B COTTON, AND R C G RUSSELL From the Departments ofSurgery, Gastroenterology and Medicine, The Middlesex Hospital and Medical School, London SUMMARY The operation oftotal pancreatectomy is performed rarely. Its role in the management of patients with chronic pancreatitis remains to be elucidated. We have reviewed our series of 29 total pancreatectomies for benign disease [14 women median age 39 years; 15 men median age 34 years]. Twelve underwent standard total pancreatectomy, in 17 duodenum preserving total pancreatectomy (DPTP) was performed. There was one death (mortality 3.4%). In no patient was the total pancreatectomy the first operative procedure. The patients were compared with age and sex matched diabetic control subjects selected on a best fit basis from the diabetic clinic database. The aetliology of the pancreatitis was idiopathic nine, pancreas divisum nine, alcohol eight and other causes three. The indication for surgery was pain 27, acute pancreatitis one and cholangitis with pancreatitis one. The complications ofthe procedures were mainly caused by infection [wound three, chest six and central line sepsis four] and in two there was a leak from the duodenum; no patient required re-operation. The postoperative stay [standard total, median 21 days (range 13-98) DPTP median 31 days (range 17-49)] has lengthened over the period due to greater attention to analgesic, diabetic and enzyme deficiency control before discharge. -

Table of Contents
DISCLAIMER The information contained within this document is restricted to and intended for professional use by licensed health care providers. The statements made should not be construed as a claim, nor does it represent any particular product procedure or advice as constituting a specific cure, whether palliative or ameliorative. Procedures and products mentioned are designed to support the client’s health and may be considered as support for other procedures deemed necessary by the practitioner. Although specific manufacturers make the supplements, herbs and homeopathies referenced in this document, the opinions expressed are those of the author and are not those of any of the manufacturers. This document is not designed to replace medical advice or medical treatments, but to be used adjunctively in the care and support of health and vitality. 9 © Copyright 2005 Return to Table of Contents . CHAPTER II: SUGAR HANDLING BLOOD SUGAR STABILIZATION RELATED CONDITIONS • Hypoglycemia PROTOCOL AT A GLANCE • Reactive Hypoglycemia • Low blood sugar Primary Supplemental Support • Syndrome X Bio-Glycozyme Forte • Triglycerides high Biomega-3 • Carbohydrate sensitivity Secondary Supplemental Support • Pre-diabetic Whey Protein Isolate • B vitamin deficiency Amino Acid Quick Sorb • Sugar sensitivity Beta-TCP • Food cravings ADHS Cytozyme-PAN • Carbohydrate cravings Cytozyme-LV Cytozyme-AD PHYSIOLOGIC CONSIDERATIONS Tri-Chol Blood sugar problems begin in childhood with excess use of sugary snacks and drinks. This is extremely common today. The U.S. Department of Agriculture estimates that the average American consumes from 150 to 180 pounds of sugar each year––an unbelievable amount compared to two or three hundred years ago when normal sugar consumption was one to five pounds per year. -

Gastroparesis and Dumping Syndrome: Current Concepts and Management
Journal of Clinical Medicine Review Gastroparesis and Dumping Syndrome: Current Concepts and Management Stephan R. Vavricka 1,2,* and Thomas Greuter 2 1 Center of Gastroenterology and Hepatology, CH-8048 Zurich, Switzerland 2 Department of Gastroenterology and Hepatology, University Hospital Zurich, CH-8091 Zurich, Switzerland * Correspondence: [email protected] Received: 21 June 2019; Accepted: 23 July 2019; Published: 29 July 2019 Abstract: Gastroparesis and dumping syndrome both evolve from a disturbed gastric emptying mechanism. Although gastroparesis results from delayed gastric emptying and dumping syndrome from accelerated emptying of the stomach, the two entities share several similarities among which are an underestimated prevalence, considerable impairment of quality of life, the need for a multidisciplinary team setting, and a step-up treatment approach. In the following review, we will present an overview of the most important clinical aspects of gastroparesis and dumping syndrome including epidemiology, pathophysiology, presentation, and diagnostics. Finally, we highlight promising therapeutic options that might be available in the future. Keywords: gastroparesis; dumping syndrome; pathophysiology; clinical presentation; treatment 1. Introduction Gastroparesis and dumping syndrome both evolve from a disturbed gastric emptying mechanism. While gastroparesis results from significantly delayed gastric emptying, dumping syndrome is a consequence of increased flux of food into the small bowel [1,2]. The two entities share several important similarities: (i) gastroparesis and dumping syndrome are frequent, but also frequently overlooked; (ii) they affect patient’s quality of life considerably due to possibly debilitating symptoms; (iii) patients should be taken care of within a multidisciplinary team setting; and (iv) treatment should follow a step-up approach from dietary modifications and patient education to pharmacological interventions and, finally, surgical procedures and/or enteral feeding. -

Neurologic Manifestations of Hypoglycemia
13 Neurologic Manifestations of Hypoglycemia William P. Neil and Thomas M. Hemmen University of California, San Diego United States of America (USA) 1. Introduction Unlike most other body tissues, the brain requires a continuous supply of glucose. It has very limited endogenous glycogen stores, and does not produce glucose intrinsically.1 Although it accounts for 2% of body weight, the brain utilizes 25% of the body’s glucose due to its high metabolic rate.2, 3 Evidence for the brains sole reliance on glucose came from obtaining a respiratory quotient of one after measuring differences between arterial and venous content of oxygen and carbon dioxide in blood traveling through the brain.4 In the past, neurons were thought to directly metabolize glucose, however, more recent studies suggest astrocytes may play an important role in glucose metabolism.5 Astrocytic foot processes surround brain capillaries, which deliver glucose to the brain. With this, they form the first cellular barrier for entering glucose.5 Astrocytes contain the non-insulin dependent GLUT1 transporter, as well as the insulin dependent GLUT4 transporter, suggesting a possible role for astrocytes in regulating and storing brain glucose in an insulin dependent and independent manner (see figure 1).6-8 In addition to glucose, the brain contains a very limited store of glycogen, (between 0.5 and 1.5 g, or about 0.1% of total brain weight). Unlike peripheral tissue, where glycogen is readily mobilized during hypoglycemia, the brain can only function normally for a limited duration. Glycogen content seems to fall in areas of highest brain metabolic rate, suggesting at least some, albeit limited role as fuel during hypoglycemia.7 Although the brain relies primarily on glucose during normal conditions, it can use ketone bodies during starvation. -

1 Jasvinder Chawla, MD, MBA Chief Neurology, Hines Veterans Affairs
Jasvinder Chawla, MD, MBA Chief Neurology, Hines Veterans Affairs Hospital, Professor of Neurology, Loyola University Medical Medical Center Jasvinder Chawla, MD, MBA is a member of the following medical societies: American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, American Clinical Neurophysiology Society, American Medical Association. Specialty Editor Board Francisco Talavera, PharmD, PhD Adjuct Assistant Professor, University of Nebraska Medical Center College of Pharmacy, Editor-in-Chief, Medscape Drug Reference Howard S Kirshner, MD Professor of Neurology, Psychiatry and Hearing and Speech Sciences, Vice Chairman, Department of Neurology, Vanderbilt University School of Medicine, Director, Vanderbilt Stroke Center, Program Director, Stroke Service, Vanderbilt Stallworth Rehabilitation Hospital, Consulting Staff, Department of Neurology, Nashville Veterans Affairs Medical Center. Chief Editor Helmi L Letsep, MD Professor and Vice Chair, Department of Neurology, Oregon Health and Science University School of Medicine, Associate Director, OHSU Stoke Center Additional Contributors Pitchaiah Mandava, MD, PhD Assistant Professor, Department of Neurology, Baylor College of Medicine, Consulting Staff, Department of Neurology, Michael E DeBakey Veterans Affairs Medical Center. Richard M Zweifler, MD Chief of Neurosciences, Sentara Healthcare, Professor and Chair of Neurology, Eastern Virginia Medical School. 1 Thomas A Kent, MD Professor and Director of Stroke Research and Education, Department -

Blood Sugar the Hidden Factor in Health
Blood Sugar The Hidden Factor in Health Disorders in blood sugar balance disrupt all aspects of human physiology. To understand this, we must keep in mind that our bodies primarily produce their energy from converting glucose (blood sugar) into ATP. If this system is not working properly, health cannot be achieved. Blood sugar disorders are extraordinarily common in the U.S. today. Much (but not all) of the prob- lem can be placed on the Standard American Diet that is high in saturated fats and sugars while being low in essential fatty acids and fiber. In the past decade, there has been an explosion in the number of cases of diabetes, which only figures to grow in the decades ahead. Insulin Insulin is a protein hormone secreted by the pancreas. Its primary job is to stimulate the uptake of glucose from our blood into our cells. Cell membranes have a lipid layer thru which glucose cannot pass on its own. It has to be carried across with the assistance of insulin. Once inside the cells, glu- cose can be used for energy. All foods are ultimately converted into glucose. An increase of glucose in the bloodstream stimulates the release of more insulin. Insulin promotes the production of glycogen, which is the form that glucose is stored in, for later use. Insulin also promotes the formation of lipids, triglyceride and protein. Alterations in insulin are responsible for causing metabolic disorders such as hypoglycemia and diabetes. Hypoglycemia If the pancreas overreacts to a sudden surge in glucose, it will release excess insulin which will subsequently cause a drop in blood sugar. -
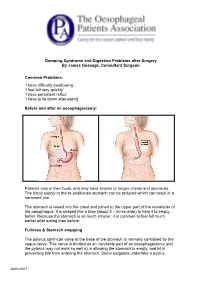
Dumping Syndrome and Digestion Problems After Surgery by James Gossage, Consultant Surgeon Common Problems: 'I Have Difficulty
Dumping Syndrome and Digestion Problems after Surgery By James Gossage, Consultant Surgeon Common Problems: ‘I have difficulty swallowing’. ‘I feel full very quickly’ ‘I have persistent reflux’ ‘I have to lie down after eating’ Before and after an oesophagectomy: Patients vary in their build, and may have shorter or longer chests and stomachs. The blood supply to the re-positioned stomach can be reduced which can result in a narrowed join. The stomach is raised into the chest and joined to the upper part of the remainder of the oesophagus. It is shaped like a tube (about 5 – 6cms wide) to help it to empty better. Because the stomach is so much smaller, it is common to feel full much earlier after eating than before. Fullness & Stomach emptying The pylorus sphincter valve at the base of the stomach is normally controlled by the vagus nerve. This nerve is divided as an inevitable part of an oesophagectomy and the pylorus may not work so well a) in allowing the stomach to empty, and b) in preventing bile from entering the stomach. Some surgeons undertake a pyloric 26/01/2017 myotomy (cutting the muscles to increase its capacity) as part of the main surgery; others prefer to wait and see if a problem develops, and then treat it by stretching it with an endoscope. Difficulty in Swallowing: Difficulty in Swallowing Narrow join Acid reflux leading to inflammation Fungal infection (candidiasis) Treatment options can be: Endoscopic dilation – acid suppression – sucralfate (binds the stomach) – antifungals Motility agents (domperidone, metoclopramide (good anti-sickness medication but needs 6 weeks to be fully effective; erythromycin). -

Gastrointestinal Motility Disorders
Gastrointestinal Motility Disorders Jassin M. Jouria, MD Dr. Jassin M. Jouria is a medical doctor, professor of academic medicine, and medical author. He graduated from Ross University School of Medicine and has completed his clinical clerkship training in various teaching hospitals throughout New York, including King’s County Hospital Center and Brookdale Medical Center, among others. Dr. Jouria has passed all USMLE medical board exams, and has served as a test prep tutor and instructor for Kaplan. He has developed several medical courses and curricula for a variety of educational institutions. Dr. Jouria has also served on multiple levels in the academic field including faculty member and Department Chair. Dr. Jouria continues to serve as a Subject Matter Expert for several continuing education organizations covering multiple basic medical sciences. He has also developed several continuing medical education courses covering various topics in clinical medicine. Recently, Dr. Jouria has been contracted by the University of Miami/Jackson Memorial Hospital’s Department of Surgery to develop an e-module training series for trauma patient management. Dr. Jouria is currently authoring an academic textbook on Human Anatomy & Physiology. Abstract The muscles of the gastrointestinal (GI) tract perform an important job. The GI tract peristalsis, or contractions, mix the contents of the stomach and propel contents throughout the entire GI tract until they exit as waste. When these muscles underperform or fail to perform, it can create serious and painful consequences, diagnosed as GI motility disorders. Although these disorders are rarely fatal, they can cause physical and emotional effects that negatively impact a patient's quality of life. -

Ocreotide and Its Effects on Steroid Resistant Crohn's Disease
2ND YEAR RESEARCH ELECTIVE RESIDENT’S JOURNAL Volume III, 1998-1999 Octreotide and its effects on Steroid resistant Crohn’s Disease Bonnie Wolf A. Introduction The treatment of Crohn’s disease continues to be a challenge as its pathogenesis remains obscure. Compounded by the increased incidence of Crohn's disease in the past twenty years incidence of 5/100,000, prevalence of 50/100,000), we are faced with an ever increasing number of treatment failures. The pathogenesis of inflammatory bowel disease stems from a dysregulation of the immune system, with persistent amplification of inflammatory cytokines, ultimately leading to tissue destruction and disease. Conventional treatment continues to be sulphasalazine or 5-ASA and glucocorticoids. For early disease and maintenance therapy, these medications have proven to be satisfactory and widely tolerated. Yet by the nature of this disease, patients relapse. They require increasing doses of medications thereby becoming resistant and simultaneously developing side effects. Sulphasalazine was shown to be more effective than placebo in controlling active disease, but was not as efficacious as steroids (2,3). In addition, not all patients can tolerate 5-ASA secondary to mild side effects including diarrhea, dizziness, nausea, and headache. Glucocorticoids are still considered to be very effective and are the most popular prescribed medications for acute active disease. The National Cooperative Crohn’s Disease Study showed that 47% of patients achieved remission and 60% improvement under therapy with varying doses of prednisilone. (2) Despite it effectiveness, glucocorticoids have a wide and significant side effect profile, and are considered unsafe to be used with frequent exacerbations and moreover as maintenance therapy.(1) Most recently, immunosuppressive agents such as azathioprine (Imuran) and Purinethanol (6- mercaptopurine.) have been introduced as potential therapies for patients who have chronic active disease or who are steroid dependent. -

Post-Whipple: a Practical Approach to Nutrition Management
NINFLAMMATORYUTRITION ISSUES BOWEL IN GASTROENTEROLO DISEASE: A PRACTICALGY, SERIES APPROACH, #108 SERIES #73 Carol Rees Parrish, M.S., R.D., Series Editor Post-Whipple: A Practical Approach to Nutrition Management Nora Decher Amy Berry The classic pancreatoduodenectomy (PD), also known as the Kausch-Whipple, and the pylorus- preserving pancreatoduodenectomy (PPPD) are most commonly performed for treatment of pancreatic cancer and chronic pancreatitis. This highly complex surgery disrupts the coordination of tightly orchestrated digestive processes. This, in combination with a diseased gland, sets the patient up for nutritional complications such as altered motility (gastroparesis and dumping), pancreatic insufficiency, diabetes mellitus, nutritional deficiencies and bacterial overgrowth. Close monitoring and attention to these issues will help the clinician optimize nutritional status and help prevent potentially devastating complications. 63-year-old female, D.D., presented to the copper, zinc, selenium, and potentially thiamine University of Virginia Health System (UVAHS) (thiamine was repleted before serum levels were Awith weight loss and biliary obstruction. She was checked). A percutaneous endoscopic gastrostomy with diagnosed with a large pancreatic serous cystadenoma jejunal extension (PEG-J) was placed due to intolerance and underwent a pancreatoduodenectomy (PD) of gastric enteral nutrition (EN). After several more (standard Whipple procedure with partial gastrectomy) hospitalizations, prolonged rehabilitation in a nursing with posterior anastomosis and cholecystectomy. Seven home, 7 months of supplemental nocturnal EN, and months later she was admitted to UVAHS with nausea, treatment of pancreatic insufficiency with pancreatic vomiting, diarrhea and a severe weight loss of 47lbs enzymes (with her meals and EN), D.D. was able to (33% of her usual body weight). -

Jack L. Snitzer,D.O
JACK L. SNITZER,D.O. Internal Medicine Board Review Course 2019 ENDOCRINE PANCREAS JACK L. SNITZER, D.O. Peninsula Regional Endocrinology 1415 S. Division Street Salisbury, MD 21804 Phone:410-572-8848 Fax:410-572-6890 E-Mail: [email protected] Endocrine Pancreas n Alpha Cells: Glucagon n Beta Cells: Insulin n Delta Cells: Somatostatin n D1 Cells: Vasoactive intestinal Polypeptide (VIP) n F Cells: Pancreatic Polypeptide (PP) n G Cells: Gastrin GLUCAGONOMA -Glucagonoma Causes increased glucose production. Rare, often malignant tumors. Classic Rash: Necrolytic Migratory Erythema Painful glossitis, angular stomatitis. Tx: Surgery, Octreotide INSULINOMA -Insulinoma Spontaneous hypoglycemia. 80% are benign. Usually very small tumors. Second most common pancreatic tumor found in MEN 1. INSULINOMA -Insulinoma Dx: elevated insulin and C-peptide level with simultaneously low glucose and symptoms. -Fasting glucose <50 mg/dl. Tx: Surgery. Octreotide to palliate. Hypoglycemia: other causes in non- diabetics • Reactive hypoglycemia (generally in obese patient with metabolic syndrome) • Malnutrition (celiac disease, eating disorder, etc.) • Cortisol insufficiency (adrenal or pituitary cause) • Nesidioblastosis (for instance: post-gastric bypass: islet cell hyperplasia) Hypoglycemia: other causes in non- diabetics • Liver disease • Surreptitious, malicious or inadvertent use of insulin. In this case, when the BG is low, insulin level will be high and C-peptide level will be low. Need to obtain these levels before treating with glucose or glucagon. • Surreptitious use of insulin secretagogue. Insulin and c- peptide levels will be high when BG is low. • Glucometer error (glucose meters are inaccurate when the BG is low); or hypoperfusion of the fingers Hypoglycemia: other causes in non- diabetics • Remember: early hypoglycemia symptoms (shakes, sweats, anxiety, hunger) are due to catecholamine release.