Cochrane Handbook for Systematic Reviews of Interventions Cochrane Handbook for Systematic Reviews of Interventions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Cochrane Collaboration Establishes Representation in Colombia Why and for What Purpose?
EDITORIAL • The Cochrane CollaborationE DITORIin ColombiaAL The Cochrane Collaboration establishes representation in Colombia Why and for what purpose? María XiMena rojas-reyes • Bogotá, D.C. (ColoMBia) DOI: https://doi.org/10.36104/amc.2020.1382 Colombia is, among Latin American countries, the one with the longest track record in the effort to introduce evidence-based medicine to patient care and, subsequently, base health policy decisions on the evidence obtained from research. Several universities have contributed to this process, beginning with the creation of the first master’s program in clinical epidemiology in April 1997 at the Pontificia Universidad Javeriana School of Medicine, followed by an interdepartmental master´s program in clinical epidemiology at the Universidad Nacional approved in 2004, and a clinical epidemiology program affiliated with the Universidad de Antioquia School of Medicine begun in 2005, and continuing to where we are today, with more than six specialization and master’s programs in clinical epidemiology offered at various universities throughout the country. Clinical epidemiology is a discipline in which scientific observations of intact human beings can be carried out and interpreted through the application of epidemiological meth- ods and principles to clinical practice problems. It provides clinicians with information regarding basic research methods which allows them to not only understand and assimilate the information from studies published in the literature, but also to organize their own observations to extract them from the anecdotal level and constitute them as scientifically solid, methodologically valid and clinically relevant assertions. Thus, the clinical epidemiology training programs have contributed to a growing number of clinicians nationwide having the ability to evaluate the validity of information in the medical literature (based on which patient care decisions are made) and produce a synthesis of valid and relevant information to guide the approach to and management of clinical problems. -

The Cochrane Afraid to Challenge the Diagnostic Acu- Men Ofhis Ancestors Or Peers
place or at the wrong time. He was not The Cochrane afraid to challenge the diagnostic acu- men ofhis ancestors or peers. He Collaboration believed that clinical questions often were answered on the basis oftests, Lessons for Public Health rather than on common sense. Practice and Evaluation? Obstetrics offered Cochrane an example ofthe practices ofthe day. Like many other fields ofmedicine, MIRUAM ORLEANS, PHD obstetrics adhered to treatments that perhaps were oftraditional or emo- tional value but which had little basis Archie Cochrane undoubtedly in science. The therapeutic use ofiron wanted to reach providers of and vitamins, the basis for extended health care with his ideas, but he lengths ofstay in hospitals following probably never thought that he would childbirth, and the basis for deciding father a revolution in the evaluation of how many maternity beds were needed medical practices. in Britain were all questioned by In his book ofonly 92 pages, Cochrane, who believed that these "Effectiveness and Efficiency: Random matters could and should be investi- Reflections on Health Services," pub- gated in trials. lished by the Nuffield Provincial Hos- Although Cochrane was by no pitals Trust in 1972, he cast a critical means the first clinician-epidemiologist eye on health care delivery, on many to suggest that randomized controlled A. L Cochrane well-respected and broadly applied trials were an appropriate means of interventions, and on whole fields of deciding questions regarding the effi- were appropriate for laboratory studies medicine and their underlying belief ciency and benefit oftreatment, I can and probably some animal and behav- systems (1). -

CRD Guidance for Undertaking Systematic Reviews in Health Care
CRD’s original guidance for undertaking systematic reviews was first published in 1996 and revised in 2001. The guidance is widely used, both nationally and internationally. The purpose of this third updated and expanded edition remains to provide practical guidance for undertaking systematic reviews evaluating the effects of health interventions. It presents the different stages of the process and incorporates issues specific to reviews of diagnostic and prognostic tests, public health interventions, adverse effects and economic evaluations. Recognising that health care decision-making often involves complex questions that go beyond ‘does it work’, the guidance also includes information relating to how and why an intervention works. CRD is part of the National Institute for Health Research (NIHR) and is a department of the University of York, UK. CRD was established in 1994 and undertakes systematic reviews evaluating the effects of interventions used in health and social care. ISBN 978-1-900991-19-3 Centre for Reviews and Dissemination University of York Heslington SAMPLE York YO10 5DD United Kingdom 9 781900 991193 www.york.ac.uk/inst/crd Systematic Reviews CRD’s guidance for undertaking reviews in health care CCRDRD SSystematicystematic RReviews.inddeviews.indd 228383 88/1/09/1/09 009:29:569:29:56 © Centre for Reviews and Dissemination, University of York, 2008 Published by CRD, University of York January 2009 ISBN 978-1-900640-47-3 This publication presents independent guidance produced by the Centre for Reviews and Dissemination (CRD). The views expressed in this publication are those of CRD and not necessarily those of the NHS, the NIHR or the Department of Health. -

The Cochrane Collaboration: an Introduction P
toolbox research are unlikely to have a high ticular research questions. This is why become dated and irrelevant. Because of number of clinically based trials direct the evidence-based supplement will also this we will seek to both review our core ed towards them. Equally journals endeavour to include these when appro list regularly and also look beyond this which publish qualitative research are priate and well conducted. list. If any reader feels any particular disadvantaged in a table based on ran It is equally important that only well article is important and should be domised-controlled trials or systematic conducted and reported randomised included please feel free to contact the review. However rating journals on the controlled trials or systematic reviews editorial office with the reference. We basis of their number of citations is are included. If we were to look in detail will then include it in our review equally open to challenge as noted at both the randomised-controlled tri process. Provided it passes the quality above. als and systematic reviews identified by filters it will appear in the supplement. Well-conducted randomised -con- the search strategy above many would trolled trials and systematic reviews not meet our quality criteria (see page Acknowledgements based on them have great potential for 32). This supplement will endeavour to To Christine Allot, Librarian, at the Berkshire Health Authority who conducted the answering questions about whether a include only good quality articles relat medline searches. treatment does more harm than good ing to treatment, diagnostic testing, particularly in the clinical situation. -

SURE Guides for Preparing and Using Evidence-Based Policy Briefs 1
SURE Guides for Preparing and Using Evidence-Based Policy Briefs 1. Getting started Version 2.1 – Updated November 2011 The SURE Collaboration Suggested citation The SURE Collaboration. SURE Guides for Preparing and Using Evidence-Based Policy Briefs: 1. Getting started. Version 2.1 [updated November 2011]. The SURE Collaboration, 2011. Available from www.evipnet.org/sure Copyright You are free to use, distribute and reproduce this work provided the source is properly cited. You may not use this work for commercial purposes without the permission of the SURE Collaboration. SURE is a collaborative project that builds on and supports the Evidence-Informed Policy Network (EVIPNet) in Africa and the Regional East African Community Health (REACH) Policy Initiative. The project involves teams of researchers and policymakers in seven African countries and is supported by research teams in three European countries and Canada. SURE is funded by the European Commission’s 7th Framework Programme (Grant agreement no 222881). Web address: www.evipnet.org/sure Email address: [email protected] 1 1. Getting started 2 Summary In this guide we describe what an ‘evidence-based policy brief’ is, what should be included in such a document, the ways in which it can be used, and the first steps required when preparing one. • What is a policy brief? • What should be included in a policy brief? • How can policy briefs be used? • First steps This guide also includes additional resources for developing a plan to build the capacity needed to prepare and support the use of policy briefs. Resources are also provided for the preparation of rapid responses for policymakers in circumstances in which they may need research evidence but there is insufficient time to prepare a policy brief, or in instances when there is no need to prepare one. -

Evaluation of NIHR Investment in Cochrane Report
Evaluation of NIHR investment in Cochrane infrastructure and systematic reviews Committee members: Professor Jos Kleijnen (Chair), Dr Phil Alderson, Dr Jane Aubin, Professor John Cairns, Ms Sally Crowe, and Professor Paul Garner Report writer: Kate Misso 10 February 2017 1 TABLE OF CONTENTS LIST OF TABLES ............................................................................................................................ 5 LIST OF FIGURES ........................................................................................................................... 6 LIST OF ABBREVIATIONS ............................................................................................................... 7 EXECUTIVE SUMMARY ............................................................................................................... 10 1. The global landscape of systematic reviews ............................................................................. 10 2. The performance of NIHR funded Cochrane Review Groups (CRGs) ....................................... 11 3. Cochrane’s impact on key clinical and policy issues in the NHS ............................................... 13 4. The economic impact of systematic reviews ............................................................................ 14 5. Current and planned developments in Cochrane and stakeholders’ views ............................. 14 Conclusions ...................................................................................................................................... -

Experiences of Using Cochrane Systematic Reviews by Local HTA Units
http://ijhpm.com Int J Health Policy Manag 2020, x(x), 1–6 doi 10.34172/ijhpm.2020.133 Short Communication Experiences of Using Cochrane Systematic Reviews by Local HTA Units Thomas G. Poder1,2* ID , Marc Rhainds3 ID , Christian A. Bellemare4 ID , Simon Deblois5 ID , Imane Hammana5 ID , Catherine Safianyk6 ID , Sylvie St-Jacques6 ID , Pierre Dagenais7,8 ID Abstract Article History: This study evaluated the use of Cochrane systematic reviews (CSRs) by Quebec’s local health technology assessment (HTA) Received: 21 November 2019 units to promote efficiency in hospital decision-making. An online survey was conducted to examine: Characteristics of Accepted: 13 July 2020 the HTA units; Knowledge about works and services from the Cochrane Collaboration; Level of satisfaction about the ePublished: 1 August 2020 use of CSRs; Facilitating factors and barriers to the implementation of CSRs evidence in a local context; Suggestions to improve the use of CSRs. Data accuracy was checked by 2 independent evaluators. Ten HTA units participated. From their implementation a total of 321 HTA reports were published (49.8% included a SR). Works and services provided by the Cochrane collaboration were very well-known and HTA units were highly satisfied with CSRs (80%-100%). As regards to applicability in HTA and use of CSRs, major strengths were as follow: Useful as resource for search terms and background material; May reduce the workload (eg, brief review instead of full SR); Use to update a current review. Major weaknesses were: Limited use since no CSRs were available for many HTA projects; Difficulty to apply findings to local context; Focused only on efficacy and innocuity; Cannot be used as a substitute to a full HTA report. -
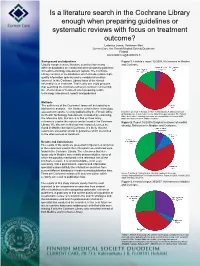
Is a Literature Search in the Cochrane Library Enough When Preparing
Is a literature search in the Cochrane Library enough when preparing guidelines or systematic reviews with focus on treatment outcome? Lodenius Leena, Honkanen Mari Current Care, the Finnish Medical Society Duodecim Finland [email protected] Background and objectives Figure 1. Finohta’s report 32/2008. References in Medline Usually comprehensive literature searches from many and Cochrane. different databases are conducted when preparing guidelines or health technology assessment reports. The Cochrane Library consists of six databases which include reliable high- quality information (primary and secondary information sources). In the Cochrane Library focus of the clinical information is in treatment. That is why one could presume that searching the Cochrane Library is sufficient concerning the effectiveness of treatment when preparing health technology assessment reports and guidelines. Methods The sufficiency of the Cochrane Library will be tested by a bibliometric analysis. The literature used in three technology assessment reports, recently published by the Finnish Office Ryynänen OP, Iirola T, Reitala J, Pälve H, Malmivaara A. Effectiveness of prehospital care. A systematic review. Finohta’s report 32/2008. The Finnish for Health Technology Assessment, is studied by examining Office for Health Technology Assessment. Finohta/Stakes, Helsinki 2008. the reference lists. Our aim is to find out how many ISBN 978-951-33-2200-7. ISSN 1239-6273. references used in the reports can be found in the Cochrane Figure 2. Report 16/2009 (Surgical treatment of morbid Library. We also aim to find out how many references are obesity). References in Medline and Cochrane. found in Medline but not in Cochrane. It´s likely, that the results are somewhat similar in guidelines which are limited to the effectiveness of treatment. -

The Cochrane Collaboration: Making the Results of Controlled Trials Properly Accessible Andrew Herxheimer
Postgrad Med J (1993) 69, 867 - 868 Postgrad Med J: first published as 10.1136/pgmj.69.817.867 on 1 November 1993. Downloaded from Special Article The Cochrane Collaboration: making the results of controlled trials properly accessible Andrew Herxheimer Cochrane Centre, Summertown Pavilion, Middle Way, Oxford OX2 7LG, UK The Cochrane Centre in Oxford was officially at Oxford University, the Department of Clinical opened in November 1992, and is perhaps the most Epidemiology and Biostatistics at McMaster visible part so far ofthe recently launched National University, and The Online Journal of Current Health Service Research and Development Pro- Clinical Trials2 published by the American gramme. Its task is to facilitate and extend the Association for the Advancement of Science. creation of systematic reviews of randomized con- trolled trials (RCTs) evaluating health care. It is named after Archie Cochrane (1909-1988), the How the Cochrane Centres are supporting the epidemiologist who first emphasized that reliable Collaboration information from RCTs, together with other essen- tial information, is vital for making sound The time needed to prepare valid reviews of RCTs Protected by copyright. decisions in health care and research. Most tends to be grossly underestimated. Lack of clinicians and clinical scientists who have tried to experience and time often force good scientists to review the evidence for a particular procedure or produce scientifically inadequate reviews. The treatment have felt frustrated by the difficulties of Cochrane Centres will give practical support to finding out what RCTs have been done, and of those preparing and updating reviews within the interpreting their results critically. -

Process of Information Retrieval for Systematic Reviews and Health Technology Assessments on Clinical Effectiveness
1 2 GUIDELINE Process of information retrieval for systematic reviews and health technology assessments on clinical effectiveness Version 1.1, December 2016 The primary objective of EUnetHTA scientific guidance is to focus on methodological challenges that are encountered by HTA assessors while performing relative effectiveness assessments of pharmaceuticals or non-pharmaceutical health technologies. As such the guideline represents a consolidated view of non-binding recommendations of EUnetHTA network members and in no case an official opinion of the participating institutions or individuals. Version Description of changes Funding Date of publication 1.0 First Version EUnetHTA JA2 July 2015 WP7 1.1 Editorial changes and minor updates EUnetHTA JA3 December 2016 based on recently published literature. WP6 The meaning of recommendations was not altered. European network for Health Technology Assessment | www.eunethta.eu Copyright © EUnetHTA 2016. All Rights Reserved. No part of this document may be reproduced without an explicit acknowledgement of the source and EUnetHTA’s expressed consent - 2 - This methodological guideline on “Process of information retrieval for systematic reviews and health technology assessments on clinical effectiveness” has been developed by the Institute for Quality and Efficiency in Health Care (IQWiG) / Germany Contact person: Elke Hausner ([email protected]) The version 1.0 of this guideline was reviewed by a group of dedicated reviewers from: • National Institute for Health and Care Excellence (NICE) / United Kingdom • State Health Care Accreditation Agency (VASPVT) / Lithuania The version 1.1 of this guideline was reviewed by a draft group with members from: • Norwegian Institute of Public Health (NIPHNO) / Norway • Andalusian Agency for Health Technology Assessment (AETSA) / Spain Copyright © EUnetHTA 2016. -

Forecasting for COVID-19 Has Failed John PA Ioannidis
Forecasting for COVID-19 has failed John P.A. Ioannidis (1), Sally Cripps (2), Martin A. Tanner (3) (1) Stanford Prevention Research Center, Department of Medicine, and Departments of Epidemiology and Population Health, of Biomedical Data Science, and of Statistics, Stanford University, and Meta-Research Innovation Center at Stanford (METRICS), Stanford, California, USA (2) School of Mathematics and Statistics, The University of Sydney and Data Analytics for Resources and Environments (DARE) Australian Research Council, Sydney, Australia (3) Department of Statistics, Northwestern University, Evanston, Illinois, USA Correspondence to: John P.A. Ioannidis, MD, DSc, Stanford Prevention Research Center, 1265 Welch Road, Medical School Office Building, Room X306, USA. E-mail: [email protected] 1 ABSTRACT Epidemic forecasting has a dubious track-record, and its failures became more prominent with COVID-19. Poor data input, wrong modeling assumptions, high sensitivity of estimates, lack of incorporation of epidemiological features, poor past evidence on effects of available interventions, lack of transparency, errors, lack of determinacy, looking at only one or a few dimensions of the problem at hand, lack of expertise in crucial disciplines, groupthink and bandwagon effects and selective reporting are some of the causes of these failures. Nevertheless, epidemic forecasting is unlikely to be abandoned. Some (but not all) of these problems can be fixed. Careful modeling of predictive distributions rather than focusing on point estimates, considering multiple dimensions of impact, and continuously reappraising models based on their validated performance may help. If extreme values are considered, extremes should be considered for the consequences of multiple dimensions of impact so as to continuously calibrate predictive insights and decision-making. -

Systematic Review of the Effectiveness of Training Programs in Writing For
Galipeau et al. Systematic Reviews 2013, 2:41 http://www.systematicreviewsjournal.com/content/2/1/41 PROTOCOL Open Access Systematic review of the effectiveness of training programs in writing for scholarly publication, journal editing, and manuscript peer review (protocol) James Galipeau1*, David Moher1,2, Becky Skidmore3, Craig Campbell4, Paul Hendry2, D William Cameron1,2, Paul C Hébert1,2 and Anita Palepu5 Abstract Background: An estimated $100 billion is lost to ‘waste’ in biomedical research globally, annually, much of which comes from the poor quality of published research. One area of waste involves bias in reporting research, which compromises the usability of published reports. In response, there has been an upsurge in interest and research in the scientific process of writing, editing, peer reviewing, and publishing (that is, journalology) of biomedical research. One reason for bias in reporting and the problem of unusable reports could be due to authors lacking knowledge or engaging in questionable practices while designing, conducting, or reporting their research. Another might be that the peer review process for journal publication has serious flaws, including possibly being ineffective, and having poorly trained and poorly motivated reviewers. Similarly, many journal editors have limited knowledge related to publication ethics. This can ultimately have a negative impact on the healthcare system. There have been repeated calls for better, more numerous training opportunities in writing for publication, peer review, and publishing. However, little research has taken stock of journalology training opportunities or evaluations of their effectiveness. Methods: We will conduct a systematic review to synthesize studies that evaluate the effectiveness of training programs in journalology.