Phelsuma 19.Indd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Distribution and Habitat of the Invasive Giant Day Gecko Phelsuma Grandis Gray 1870
Phelsuma 22 (2014); 13-28 Distribution and habitat of the invasive giant day gecko Phelsuma grandis Gray 1870 (Sauria: Gekkonidae) in Reunion Island, and conservation implication Mickaël Sanchez1,* and Jean-Michel Probst1,2 1 Nature Océan Indien, 6, Lotissement les Magnolias, Rivière des Roches, 97470 Saint-Benoît, La Réunion, France 2 Nature et Patrimoine, 2, Allée Mangaron, Dos d’Ane, 97419 La Possession, La Réunion, France * Corresponding author; e-mail: [email protected] Abstract. The giant day gecko Phelsuma grandis, endemic to Madagascar, was introduced to Reunion Island (Indian Ocean) in the mid-1990s. No studies have been conducted so far to define its precise distribution and habitat. To fill the knowledge gap about this invasive species, we compiled available data and performed field work during 2007-2014. We detected 13 distinct populations of P. grandis, occurring mainly in the northern part of Reunion Island and on the west coast. This gecko inhabits human disturbed areas (gardens, urban parks, bamboos, orchards, coconuts, and banana plantation) and secondary habitats (shrubby savanna, and secondary dry woodlands, secondary dry and wet thickets). Its distribution strongly suggests that saltatory dispersal (through deliberate and/or accidental transport) and natural colonization are the mechanisms of spreading through Reunion Island. All our data in combination with both P. grandis ecology and native environmental range suggested that this gecko may colonize native forest, and constitutes a potential important threat to the native biodiversity of Reunion Island (arthropods and lizards). Key words. Phelsuma grandis, Reunion Island, distribution, invasive species, conservation. Introduction Day geckos of the genus Phelsuma are distributed in the western Indian Ocean (Austin et al. -

The Gold Dust Or Flat Tailed Day Gecko)
British Herpetological Society Bulletin, No. 57, 1996 NOTES ON KEEPING AND BREEDING PHELSUMA LATICAUDA (THE GOLD DUST OR FLAT TAILED DAY GECKO) RICHARD HARLING 38 Twin Oaks Close, Broadstone, Dorset, BH18 8JF INTRODUCTION Gold Dust Geckoes are from the Comoros islands off Madagascar, as well as being introduced to other places including Madagascar itself. They are tropical lizards with an insectivorous diet, supplemented by fruit. During Easter 1995, I obtained three day geckos from a reptile shop in London. I wanted two females and a male but was unfortunately sent two males and a female - this I suspected quite quickly as the fully grown male would not tolerate one of the "females". "She" has now grown and become as big as him, has obvious femoral pores (always much more obvious that on the female) and is definitely a "him". It is generally recommended that captive-bred reptiles are best as they are tamer and more accustomed to captivity. DESCRIPTION In good light they are like living jewels. They have two horizontal bright red stripes across the top of their fairly blunt snouts up to their beautiful blue almost eye-shadowed eyes which do not have eyelids and are licked clean by their long pink tongue. There is often another red bar just behind the eyes. The posterior dorsal surface of the head and anterior half of the back is powdered with a yellowy-gold. Briefly the back is just green and then there are three bright red, "tear drops" which break up posteriorly into numerous small red spots which spread down the yellowy tail with a hint of green. -

On the Andaman and Nicobar Islands, Bay of Bengal
Herpetology Notes, volume 13: 631-637 (2020) (published online on 05 August 2020) An update to species distribution records of geckos (Reptilia: Squamata: Gekkonidae) on the Andaman and Nicobar Islands, Bay of Bengal Ashwini V. Mohan1,2,* The Andaman and Nicobar Islands are rifted arc-raft of 2004, and human-mediated transport can introduce continental islands (Ali, 2018). Andaman and Nicobar additional species to these islands (Chandramouli, 2015). Islands together form the largest archipelago in the In this study, I provide an update for the occurrence Bay of Bengal and a high proportion of terrestrial and distribution of species in the family Gekkonidae herpetofauna on these islands are endemic (Das, 1999). (geckos) on the Andaman and Nicobar Islands. Although often lumped together, the Andamans and Nicobars are distinct from each other in their floral Materials and Methods and faunal species communities and are geographically Teams consisted of between 2–4 members and we separated by the 10° Channel. Several studies have conducted opportunistic visual encounter surveys in shed light on distribution, density and taxonomic accessible forested and human-modified areas, both aspects of terrestrial herpetofauna on these islands during daylight hours and post-sunset. These surveys (e.g., Das, 1999; Chandramouli, 2016; Harikrishnan were carried out specifically for geckos between and Vasudevan, 2018), assessed genetic diversity November 2016 and May 2017, this period overlapped across island populations (Mohan et al., 2018), studied with the north-east monsoon and summer seasons in the impacts of introduced species on herpetofauna these islands. A total of 16 islands in the Andaman and and biodiversity (e.g., Mohanty et al., 2016a, 2019), Nicobar archipelagos (Fig. -

Phelsumaresearch ARTICLES Andamanensis Blyth 1861
WWW.IRCF.ORG/REPTILESANDAMPHIBIANSJOURNALTABLE OF CONTENTS IRCF REPTILES & AMPHIBIANSIRCF REPTILES • VOL15, &NO AMPHIBIANS 4 • DEC 2008 189 • 27(1):54–55 • APR 2020 IRCF REPTILES & AMPHIBIANS CONSERVATION AND NATURAL HISTORY TABLE OF CONTENTS FEATURE ARTICLES Notes. Chasing on Bullsnakes Courtship (Pituophis catenifer sayi) in Wisconsin: and Breeding Behavior On the Road to Understanding the Ecology and Conservation of the Midwest’s Giant Serpent ...................... Joshua M. Kapfer 190 . The Sharedof History theof Treeboas ( CorallusAndaman grenadensis) and Humans on Grenada: Day Gecko, A Hypothetical Excursion ............................................................................................................................Robert W. Henderson 198 PhelsumaRESEARCH ARTICLES andamanensis Blyth 1861 . The Texas Horned Lizard in Central and Western Texas ....................... Emily Henry, Jason Brewer, Krista Mougey, and Gad Perry 204 . The Knight Anole (Anolis equestris) in Florida (Reptilia: ............................................. Gekkonidae),Brian J. Camposano, Kenneth L. Krysko, in Kevin theM. Enge, Ellen AndamanM. Donlan, and Michael Granatosky 212Islands CONSERVATION ALERT S.R. Chandramouli . World’s Mammals in Crisis ............................................................................................................................................................. 220 Department of Ecology. andMore Environmental Than Mammals ...............................................................................................................................Sciences, -

1 7 an Identification Key to the Geckos of the Seychelles
HERPETOLOGICAL JOURNAL. Vol. I. pp. 17-19 (19X5l 17 AN IDENTIFICATION KEY TO THE GECKOS OF THE SEYCHELLES, WITH BRIEF NOTES ON THEIR DISTRIBUTIONS AND HABITS ANDREW S. GARDNER Department of Zoology, University of Aberdren. Ti/lydrone Avenue, Aberdeen AB9 2TN. U. K. Present addresses: The Calton Laboratory. Department of Genetics and Biomet IT, Universif.I' Co/legr London. Wo/f�·on !-louse, 4 Stephenson Wa r London NWI 21-11'.. U.K. (A ccepted 24. /0. 84) INTRODUCTION 4. Scales on chest and at least anterior of belly keeled. Underside white. Phe!suma astriata The Republic of Seychelles, lying in the western Tornier. 5. Indian Ocean consists of a group of mountainous, granitic islands, and a large number of outlying coral Scales on chest and belly not keeled. 6. atolls and sand cays, distributed over 400,000 km2 of sea. There are over a hundred islands, ranging in size 5. Subcaudal scales keeled and not transversely from Mahe, at 148 km2 to islands little more than enlarged in original tails. Ground colour of emergent rocks. A total of eighteen species of lizard, rump and tail usually bright blue, and of from three families are recorded from the Seychelles nanks, green. Tail unmarked or spotted with (Gardner, 1984). The best represented family is the red. Red transverse neck bars often reduced or Gekkonidae with eleven species, fo ur of which are absent. Phe/suma astriata astriata Tornier i endemic to the islands. The identification key 90 1. presented here should enable interested naturalists to Subcaudal scales unkeeled and transversely identify any gecko encountered in the Seychelles to the enlarged in original tails. -

Rediscovery of the Enigmatic Day Gecko Phelsuma Masohoala in Northeast Madagascar
Herpetological Conservation and Biology 11:402–407. Submitted: 15 April 2016; Accepted: 3 September 2016; Published: 16 December 2016. Rediscovery of the Enigmatic Day Gecko Phelsuma masohoala in Northeast Madagascar Richard C. Stanley1,3and Christopher J. Raxworthy2 1Research Associate, National Museum of Natural History, 10th Street & Constitution Avenue North West, Washington D.C. 20560, USA 2Department of Herpetology, American Museum of Natural History, Central Park West at 79th Street, New York, New York 10024-5192, USA 3Corresponding author, e-mail: [email protected] Abstract.—Phelsuma masohoala is a cryptically patterned day gecko endemic to Madagascar, and is one of the rar- est geckos known on the island. It is currently known from only four museum specimens and has not been reliably seen in the wild since 1994, despite recent attempts to find it at the type locality. Here we report the rediscovery of P. masohoala at Marojejy National Park, and review all known records of this species. Our observations provide important new data on the ecology, distribution, and behavior of this rare species, which appears to be difficult to detect due to its cryptic coloration and arboreal habits. Key Words.—conservation; distribution; Gekkonidae; Marojejy Introduction type locality, from the Tamatave area. However, doubts about the locality of this specimen have been considered Phelsuma masohoala (Raxworthy and Nussbaum sufficiently serious that it was not included in the IUCN 1994) is one of the least known species of gecko in the Red List species account (Glaw and Rakotondrazafy world. It is distinguished by its grey, black and white 2011). Photographs of this specimen in life appear in coloration; all other Phelsuma known to be sympatric Meier and Böhme (1996), Glaw and Vences (2007), and with it are primarily green. -

Madagascar Day Gecko
Madagascar Giant Day Gecko Reptile Phelsuma madagascariensis grandis Scientific Name Phelsuma madagascariensis grandis Other Names None Range Eastern Madagascar Habitat Rainforest Average Size Length: 8 – 9 inches Weight: 60 – 70 grams Description A small, mostly green gecko with red to reddish- brown spots on the body and a reddish-brown Behavior stripe from the nose to the eye. The underside As the name suggests, this lizard is diurnal (active in daytime). Males is grey. are highly territorial throughout the year, not just during breeding season, and will actively fight other males that enter their territory. They are also Diet arboreal (live in the trees) and rarely, if ever, venture to the ground, In the wild: Insects, nectar spending their days basking in the sun and hunting for insects. They move In human care: Insects easily through the trees using flattened toe pads that are covered on the bottom with dead, keratinized scales called lamellae. The lamellae scale Lifespan surface is made up of long hair-like structures called setae, with each setae In the wild: Estimated at 10 to 15 years being divided and subdivided along its length. Because of these setae, In human care: Up to 15 years these day geckos are capable of climbing up almost any surface including Incubation glass. 60 – 65 days Reproduction and Breeding Clutch Size The wild breeding season for this day gecko is in fall and early summer, Six sets of two each year generally November through March. After a brief courtship and mating, where males aggressively fight for access to mates in the area, females Sexual Maturity lay one to two eggs every four to six weeks. -

Schoenecker Et Al
Eine neue Taggecko-Art der Gattung Phelsuma aus Ost-Madagaskar Eine neue Taggecko-Art der Gattung Phelsuma aus Ost-Madagaskar (Reptilia: Squamata: Gekkonidae) PATRICK SCHÖNECKER, STEFANIE BACH & FRANK GLAW Zusammenfassung Wir beschreiben eine neue Phelsuma-Art, welche die kleinste bekannte Art der Gattung darstellt. Verwandtschaftlich steht sie vermutlich der Phelsuma lineata-Gruppe nahe. Das bisher bekannte Vorkommen beschränkt sich auf eine Region in der Umgebung von Manambato an der zentralen Ostküste Madagaskars. Schlüsselwörter: Sauria: Gekkonidae: Phelsuma; neue Art; Madagaskar. 1 Einleitung Bei der Gattung Phelsuma handelt es sich um eine artenreiche Gruppe von Taggeckos. Sie umfasst derzeit 39 Arten, die hauptsächlich auf Madagaskar und weiteren Inseln im westlichen Indischen Ozean verbreitet sind. Die Grundfarbe der Tiere setzt sich meistens aus diversen Grün- und Brauntönen zusammen, kann jedoch auch graue, gelbe, blaue, schwarze und weiße Elemente enthalten. Die Gesamtlänge der größten rezenten Arten (P. madagascariensis grandis GRAY, 1870 und P. guentheri BOULENGER, 1885) kann bis zu 300 mm betragen, wobei die bereits ausgestorbene P. gigas von der Insel Rodrigues noch erheblich größer wurde (HALLMANN et al. 1997). Die bisher kleinsten Vertreter sind P. quadriocellata parva MEIER, 1983 und P. pusilla pusilla MERTENS, 1964, die beide eine maximale Gesamtlänge von 85 mm erreichen (MERTENS 1964, HALLMANN et al. 1997). Im Jahr 2000 gelangte ein größerer Import mit madagassischen Reptilien und Amphibien nach Deutschland. Dieser enthielt unter anderem auch einige Unterarten von Phelsuma lineata (GRAY, 1842), nämlich P. l. bombetokensis MERTENS, 1964, P. l. dorsivittata MERTENS, 1964 und P. l. lineata (GRAY, 1842). Als sogenannter „Beifang“ fanden sich darunter drei auffallend kleine tagaktive Geckos. -

REPTILLIA: GEKKONIDAE) HARALD MEIER* & WOLFGANG BOEHME** Present Addresses: *Suentelstrasse 109, D-2000 Hamburg 61, F.R
British Herpetological Society Bulletin, No. 33, 1990. NOTES ON HABITAT SELECTION AND COLOURATION IN LIFE OF PHELSUMA BORBONICA AGALEGAE CHEKE, 1975 (REPTILLIA: GEKKONIDAE) HARALD MEIER* & WOLFGANG BOEHME** Present addresses: *Suentelstrasse 109, D-2000 Hamburg 61, F.R. Germany; **Department of Herpetology, Alexander Koenig Zoological Research Institute and Museum, Adenauerallee 105-164, D-5300 Bonn 1, F.R. Germany. During an excursion from Mauritius to the Seychelles A.S. Cheke had the opportunity to shortly visit Agalega, where he discovered an unknown form of Day Gecko, which he described as a new species, Phelsuma agalegae (Cheke 1975). In a subsequent paper (Cheke 1982) he raised Phelsuma cepediana borbonica Mertens, 1966, an endemic of Reunion, to specific rank and classified the Agalega population as a subspecies of the latter: P. borbonica agalegae. The senior author had the opportunity to visit Agalega from November 18 to 29, 1989, with the permission of the Ministry of Agriculture, Fisheries and Natural Resources of Mauritius, and supported by the O.I.D.C. Corporation at Port Louis. He was accompanied by Mr. Y. Mungroo as a representative of the Ministry. The results of this herpetological excursion make the following notes possible, where, however, the extensive remarks made by Cheke (1975) will not be repeated in detail. REMARKS ON HABITAT SELECTION The main study area was the village Vingt-Cinq on the northern island and its environments (Fig. 1). Vingt-Cinq consists mainly of one street with more or less scattered houses. Half- AGALEGA Seych0144 'Z' r • 0 1 2 3 4 km z 0 Maurtius Reunion() Fig. -

First Evidence of Nectarivory by Four-Clawed Gecko, Gehyra Mutilata
Herpetology Notes, volume 10: 493-496 (2017) (published online on 14 September 2017) First evidence of nectarivory by four-clawed gecko, Gehyra mutilata (Wiegmann, 1834) (Squamata: Gekkonidae) on a bat- pollinated Calabash tree (Crescentia cujete L.) (Bignoniaceae) in Southcentral Mindanao, Philippines Krizler Cejuela Tanalgo1,2,* and Alice Catherine Hughes1 In the tropics, vertebrates play an important role introduced across Asia (Burger and Gentry, 2000; as pollinators in many ecosystems. Rodents, birds, Arango-Ulloa et al., 2009). It usually grows in lowland and bats are the best-known vertebrate pollinators in and semi-evergreen tropical forests (Gentry, 1980, 1992) tropical regions (Nabhan and Buchmann, 1997). Apart and is cultivated in many gardens for aesthetic purposes from these, many lizard species have been documented and natural fences and medicines (Heinrich et al., 1998; to feed on flowers and potentially act as a pollinator Reyes and Rosado, 2000; Das et al., 2014). The Long- (Godínez-Álvarez, 2004). Yet, lizard pollination remains Nosed Bat, Glossophaga soricina (Pallas, 1766) has been understudied since the beginning of the 20th century reported as a pollinator of C. cujete in the Neotropics (Olesen and Valido, 2003). At present, there are 34 (Lemke, 1984; Lemke, 1985). In the Paleotropics, the species of lizards known to feed on nectar and flowers pollinators of this species remain understudied, but the of 97 known plant species (Godínez-Álvarez, 2004). Dagger-toothed Nectar Bat Macroglossus minimus (É. Presumably, lizards feeding on nectar are effective Geoffroy, 1810) was observed by the first author to visit pollinators because they transport pollen grains from the flowers of C. -

Taxonomic Checklist of the Day Geckos of the Genera Phelsuma Gray, 1825 and Rhoptropella Hewitt, 1937 (Squamata: Gekkonidae)
65 (2): 247 – 283 © Senckenberg Gesellschaft für Naturforschung, 2015. 23.6.2015 Taxonomic checklist of the day geckos of the genera Phelsuma Gray, 1825 and Rhoptropella Hewitt, 1937 (Squamata: Gekkonidae) compiled by Frank Glaw & Herbert Rösler at the request of the Nomenclature Specialist of the CITES Animals Committee and the German Federal Agency for Nature Conservation (BfN) Funded by the German Federal Ministry of the Environment, Nature Conservation, Building and Nuclear Safety (BMUB) 2015 65 (2): 247 – 283 © Senckenberg Gesellschaft für Naturforschung, 2015. 23.6.2015 Taxonomic checklist of the day geckos of the genera Phelsuma Gray, 1825 and Rhoptropella Hewitt, 1937 (Squamata: Gekkonidae) Frank Glaw 1 & Herbert Rösler 2 1 Zoologische Staatssammlung München (ZSM-SNSB), Münchhausenstraße 21, 81247 München, Germany; [email protected] — 2 Senckenberg Naturhistorische Sammlungen Dresden, Museum für Tierkunde, Sektion Herpetologie, Königsbrücker Landstr. 159, 01109 Dresden, Germany;[email protected] Accepted 26.5.2015. Published online at www.senckenberg.de / vertebrate-zoology on 5.6.2015. Contents Abstract ..................................................................................................................................................................... 251 Introduction ............................................................................................................................................................... 251 Collection acronyms ................................................................................................................................................ -
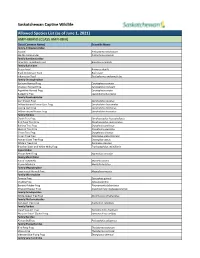
Captive Wildlife Allowed List
Saskatchewan Captive Wildlife Allowed Species List (as of June 1, 2021) AMPHIBIANS (CLASS AMPHIBIA) Class (Common Name) Scientific Name Family Ambystomatidae Axolotl Ambystoma mexicanum Marble Salamander Ambystoma opacum Family Bombinatoridae Oriental Fire-Bellied Toad Bombina orientalis Family Bufonidae Green Toad Anaxyrus debilis Black Indonesian Toad Bufo asper Indonesian Toad Duttaphrynus melanostictus Family Ceratophryidae Surinam Horned Frog Ceratophrys cornuta Chacoan Horned Frog Ceratophrys cranwelli Argentine Horned Frog Ceratophrys ornata Budgett’s Frog Lepidobatrachus laevis Family Dendrobatidae Dart Poison Frog Dendrobates auratus Yellow-banded Poison Dart Frog Dendrobates leucomelas Dyeing Dart Frog Dendrobates tinctorius Yellow-striped Poison Frog Dendrobates truncatus Family Hylidae Clown Tree Frog Dendropsophus leucophyllatus Bird Poop Tree Frog Dendropsophus marmoratus Barking Tree Frog Dryophytes gratiosus Squirrel Tree Frog Dryophytes squirellus Green Tree Frog Dryophytes cinereus Cuban Tree Frog Osteopilus septentrionalis Haitian Giant Tree Frog Osteopilus vastus White’s Tree Frog Ranoidea caerulea Brazilian Black and White Milky Frog Trachycephalus resinifictrix Hyperoliidae African Reed Frog Hyperolius concolor Family Mantellidae Baron’s Mantella Mantella baroni Brown Mantella Mantella betsileo Family Megophryidae Long-nosed Horned Frog Megophrys nasuta Family Microhylidae Tomato Frog Dyscophus guineti Chubby Frog Kaloula pulchra Banded Rubber Frog Phrynomantis bifasciatus Emerald Hopper Frog Scaphiophryne madagascariensis