Giant Extra Gastrointestinal Stromal Tumor of Lesser Omentum Obscuring
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thessaloniki Hotels Directory 2020-21
THESSALONIKI HOTELS ASSOCIATION 1 9 1 4 - 2014 THESSALONIKI HOTELS DIRECTORY 2020-21 take a stroll. in peace. this is Thessaloniki. Walk! Don’t rush. Walk. Thessaloniki’s chilled-out attitude Thessaloniki shimmers is your passport to true relaxation: admire the with its own brand magnificence of the sea while drinking a coffee of easy-living spirit -all at one of the local cafés; catch impro jazz sounds you need to do is let or alternative beats at the hip downtown bars; sample yourself go and have fun. delicious Mediterranean dishes at one of the many An impressive mix picturesque tavernas... Just take your time and enjoy! of gastronomic cultures, a rich and varied nightlife, a great history to explore on every step of the way. Do not forget to try the wines! LIVELY NIGHTLIFE City-break lovers will be amazed by the great choice of nightlife on offer, mixing traditions, cultures, the past and the present. Traditional Greek ALL KINDS music halls, live music bars, buzzing new venues showcasing the best yeah! OF TUNES live acts every night of the week, whatever you’re after, you are sure to Cozy little coffee shops and bars find it here. Have a late-night seafood feast at Perea and Aretsou by in the city centre and the Ladadika the seaside, indulge in an oriental sweet at Ano Poli (the old citadel) or port district welcome their guests wander down to Valaoritou street -the city’s latest hotspot- where you can with all kinds of tunes –from jazz to hang out with people from all walks of life till the small hours. -

GOVERNMENT GAZETTE Series B- 5922/31.12.2018
69941 GREEK GOVERNMENT GAZETTE Series B- 5922/31.12.2018 TRUE COPY 69941 OF THE ORIGINAL DOCUMENT Greek Government Gazette December 31st 2018 SERIES B Issue No. 5922 Β' 464/19-4-2010). DECISIONS 9. The provisions of ministerial decision “Natural Gas Dec. No 1314/2018 Licensing Regulation” ref. no. 178065 (Government Gazette Β' 3430/17.08.2018, hereinafter referred to as For the granting of a Natural Gas Distribution “Licensing Regulation”). License to the company under the trade name 10. The Tariffs Regulation for the Main Distribution “Gas Distribution Company Thessaloniki- Activity of distribution networks in Attica, Thessaloniki, Thessaly S.A.” and the distinctive title “EDA Thessaly and other Greece (Government Gazette Β' THESS”. 3067/26.09.2016) (hereinafter referred to as “Tariffs Regulation”). THE REGULATORY AUTHORITY FOR ENERGY 11. The RAE's Decision No 346/2016 on the Approval Taking into consideration the following: of the Tariff for the Charge of the Main Natural Gas 1. The provisions of Law 4001/2011 “For the Distribution Activity on Thessaloniki distribution network operation of the Energy Markets of Electricity and (Government Gazette Β' 3490/31.10.2016). Natural Gas, for Research, Production and transmission 12. The RAE's Decision No 347/2016 on the Approval networks of Hydrocarbons and other arrangements” of the Tariff for the Charge of the Main Natural Gas (Government Gazette A’179/22.08.2011), as amended Distribution Activity on Thessaly distribution network and in force (hereinafter referred to as “the Law”), and (Government Gazette Β'3537/03.11.2016). especially articles 13 and 80C thereof. -

Curriculum Vitae
CURRICULUM VITAE Prof. GEORGE Th. PAVLIDIS Scientific Director of the 37 “DYSLEXIA CENTERS –PAVLIDIS METHOD”, Greece, Cyprus, Serbia. PROFESSIONAL ADDRESS: ‘DYSLEXIA CENTERS – PAVLIDIS METHOD’ 72, ELPIDOS STREET, POLICHNI, THESSALONIKI 56533, GREECE Tel. 00-30-6978001844 (mobile) [email protected] FAMILY: 3 CHILDREN, ALEXANDROS, ARISTOTELIS & ODYSSEUS EDUCATION B.A.. 1972 (1st class honours) Aristotelion University of Thessaloniki, Greece. Ph.D. 1980 (Psychology) Faculty of Science, University of Manchester, England SUMMARY CV ➢ Inventor of new patented technologies and methods, recognised internationally. ➢ Internationally acknowledged authority in Dyslexia, Attention Deficit Hyperactivity Disorder (ADHD) and in Ophthalmokinesis (eye movements). ➢ Professor of Psychology and Learning Disabilities for more than 44 years in departments of Psychology, Schools of Education and in Medical Schools of known Universities in England, Greece and the USA. ➢ Visiting Professor in many English and USA Universities (Medical Schools and Schools of Education) ➢ Director of research grants in more than 12 major grants since 1975 in England, USA & Greece. ➢ Founder and Director of Dyslexia and Ophthalmokinesis Labs in universities in England, Greece and USA. ➢ Theses Supervision: Successful supervision > 25 M.Phil. and Doctoral Dissertations (Ph.D.) in England, Greece and the USA. Two (2) of them were awarded the Outstanding Dissertation of the year. ➢ 23 years old began his academic career as lecturer in psychology, in Manchester, England. ➢ 30 years -

Residences in Ano Poli (Upper Town) of Thessaloniki, Greece
JOURNAL "SUSTAINABLE DEVELOPMENT, CULTURE, TRADITIONS".................Volume 1a/2019 THE ‘KASTROPLIKTON’ RESIDENCES IN ANO POLI (UPPER TOWN) OF THESSALONIKI, GREECE. STATUS AND PROSPECTS OF PRESERVATION DOI: 10.26341/issn.2241-4002-2019-1a-3 Christina – Zoi Kefala Civil Engineer TE MSc in Historic Conservation, Oxford Brookes University Nikolaos Samaras University of Thessaly, Greece [email protected] Abstract The refugees, who came to Thessaloniki after the Minor Asia Catastrophe in 1922, according to the Treaty of Lausanne, were settled either in the abandoned Muslim residences or in new ones built in any free plot in Ano Poli of Thessaloniki. Due to the lack of space, some residences were developed along the Byzantine Wall of the City even in contact with it, using the Wall as part of the residence, making it impossible for the visitor to gain access to it, or even to see it. Since 1931, the project of the designation of the City Wall began and a Presidential Decree (Gov. Gazette 406Α΄ / 04.12.1931) was published and it was suggested the creation of a green belt alongside of the Wall. With the implementation of the Presidential Decree, about 900 houses are partially or fully demolished for the construction of the green belt. After that, the buildings of this zone are characterized as demolished and called "kastroplikton" and nowadays are divided in three categories, those that are still inhabited, those that have been abandoned and those that have already been collapsed due to lack of maintenance. Because of the designation of the City Wall, which had already been included in the UNESCO World Heritage List, new presidential decrees and a lot of studies of the local authorities were published. -

List of Cities of Greece
SNo City Census 1991 Census 2001 Census 2011 Rank Region 1 Athens 772,072 745,514 664,046 1 Attica 2 Thessaloniki 383,967 363,987 315,196 2 Central Macedonia 3 Patras 152,570 160,400 168,034 3 West Greece 4 Piraeus 182,671 175,697 163,688 4 Attica 5 Larissa 112,777 124,394 144,651 5 Thessaly 6 Heraklion 115,270 130,914 140,730 6 Crete 7 Peristeri 137,288 137,918 139,981 7 Attica 8 Kallithea 114,233 109,609 100,641 8 Attica 9 Acharnes 61,052 75,329 99,346 9 Attica 10 Kalamaria 80,698 87,255 91,279 10 Central Macedonia 11 Nikaia 87,597 93,086 89,380 11 Attica 12 Glyfada 63,306 80,409 87,305 12 Attica 13 Volos 77,192 82,439 86,046 13 Thessaly 14 Ilio 78,326 80,859 84,793 14 Attica 15 Ilioupoli 75,037 75,904 78,153 15 Attica 16 Keratsini 71,982 76,102 77,077 16 Attica 17 Evosmos 28,821 52,624 74,686 17 Central Macedonia 18 Chalandri 66,285 71,684 74,192 18 Attica 19 Nea Smyrni 69,749 73,986 73,076 19 Attica 20 Marousi 64,092 69,470 72,333 20 Attica 21 Agios Dimitrios 57,574 65,173 71,294 21 Attica 22 Zografou 80,492 76,115 71,026 22 Attica 23 Egaleo 78,563 74,046 69,946 23 Attica 24 Nea Ionia 60,635 66,017 67,134 24 Attica 25 Ioannina 56,699 61,629 65,574 25 Epirus 26 Palaio Faliro 61,371 64,759 64,021 26 Attica 27 Korydallos 63,184 67,456 63,445 27 Attica 28 Trikala 45,835 48,686 61,653 28 Thessaly 29 Vyronas 58,523 61,102 61,308 29 Attica 30 Agia Paraskevi 47,463 56,836 59,704 30 Attica 31 Galatsi 57,230 58,042 59,345 31 Attica 32 Chalcis 51,646 53,584 59,125 32 Central Greece 33 Petroupoli 38,278 48,327 58,979 33 Attica 34 Serres 50,017 -

15 Kavafi Street, Polichni, 56533, Thessaloniki, Greece
CURRICULUM VITAE PERSONAL INFORMATION: NAME: Maria SURNAME: Panagiotopoulou ADDRESS: 15 Kavafi Street, Polichni, 56533, Thessaloniki, Greece TELEPHONE: 2310-659609 MOBILE PHONE: 6977016187 E-MAIL: [email protected] PLACE OF BIRTH: Thessaloniki, Greece DATE OF BIRTH: 03.08.1978 NATIONALITY: Hellenic EDUCATION: 2002 October – 2003 September: Master of Arts (MA) in Translation Studies, University of Portsmouth, UK (grade: 2.1) 1999 October – 2002 June: : Bachelor of Arts (BA) in Modern Languages, University of Lincoln, UK (grade 2:1) LANGUAGES: . Greek (mother tongue), . English (Certificate of Proficiency in English), . German (basic knowledge in reading, writing and speaking), . Spanish (basic knowledge in reading, writing and speaking) WORKING EXPERIENCE: 2003 up until now: Freelance translator collaborating with translation agencies in Greece and abroad 2004 June – 2008 May: In-house translator, proofreader and project manager at “Oettli S.A Language Management Solutions”. Translation/proofreading of Medical, Medical-technical (medical equipment manuals, MRI, CT, AX, X-ray imaging systems, catheters, angiography, mammography, oncology, ultrasound techniques, biochemistry analyzers, IFUs, etc.) and Technical texts (computer science, machinery, tools, appliances, automotive etc.) Use of CAT tools and translation memories. The project management tasks included the management and coordination of large translation projects, preparation/conversion of files for translation, setting up/coordination of translator teams, internal QA checks -

Macedonia in Greek Administration
Preface When in 1821 Greeks started their revolt against the Ottoman Empire and fought for an independent state they had two major ideological issues to deal with: the identity of the new state and its future borders. If Hellas (Ελλάς) was the appropriate name for Modern Greece and ancient glory the most valuable argument for Greek independence, then how could Macedonia been kept apart? After all it was an integral part of Greek ancient history, which had nourished every single generation of educated people—not only Greeks—even before the war of Greek independence. The legendary figure of Alexander the Great had surfed smoothly over centuries of ignorance escorted by powerful myths and tales to find its appropriate position in the last part of 19th century, ancient history textbooks. They were the chapters of the Macedonian Hegemony and the Hellenistic period (4th and 3rd centuries B.C.), which had brought Greek culture to the frontiers of the then known world. Ancient History proved a very solid and enduring foundation for the modern Greek state. In this context, in the last quarter of the 19th century the case of Macedonia, this ill-defined region, was regarded as the final frontier of Hellenism, which Greece had to defend against the Slavs, if it was to survive as a state and not to end up as a sad caricature of Ancient Hellas. The romantic fight of the Greeks for Macedonia—in fact for the littoral part of it—created its own legend, which was shaped through a series of declared and undeclared wars from the 1878 Eastern Crisis to World War II. -

Lagarias Apostolos, Architect AUTH, PHD Candidate
A Service of Leibniz-Informationszentrum econstor Wirtschaft Leibniz Information Centre Make Your Publications Visible. zbw for Economics Lagarias, Apostolos Conference Paper Fractal Analysis of the Urbanization At the Outskirts of the City: Models, Measurement and Explanation 46th Congress of the European Regional Science Association: "Enlargement, Southern Europe and the Mediterranean", August 30th - September 3rd, 2006, Volos, Greece Provided in Cooperation with: European Regional Science Association (ERSA) Suggested Citation: Lagarias, Apostolos (2006) : Fractal Analysis of the Urbanization At the Outskirts of the City: Models, Measurement and Explanation, 46th Congress of the European Regional Science Association: "Enlargement, Southern Europe and the Mediterranean", August 30th - September 3rd, 2006, Volos, Greece, European Regional Science Association (ERSA), Louvain-la-Neuve This Version is available at: http://hdl.handle.net/10419/118541 Standard-Nutzungsbedingungen: Terms of use: Die Dokumente auf EconStor dürfen zu eigenen wissenschaftlichen Documents in EconStor may be saved and copied for your Zwecken und zum Privatgebrauch gespeichert und kopiert werden. personal and scholarly purposes. Sie dürfen die Dokumente nicht für öffentliche oder kommerzielle You are not to copy documents for public or commercial Zwecke vervielfältigen, öffentlich ausstellen, öffentlich zugänglich purposes, to exhibit the documents publicly, to make them machen, vertreiben oder anderweitig nutzen. publicly available on the internet, or to distribute or otherwise use the documents in public. Sofern die Verfasser die Dokumente unter Open-Content-Lizenzen (insbesondere CC-Lizenzen) zur Verfügung gestellt haben sollten, If the documents have been made available under an Open gelten abweichend von diesen Nutzungsbedingungen die in der dort Content Licence (especially Creative Commons Licences), you genannten Lizenz gewährten Nutzungsrechte. -

Of Social Entrepreneurship Deliverable 6.1.3
Interreg Greece-Bulgaria / Social Plate Project Action: 6.1.3 Awards for NGO participation and “champions” of social entrepreneurship Deliverable 6.1.3: Report of event Social Plate Project 1 Interreg Greece-Bulgaria / Social Plate Project Contents 1. Introduction ...................................................................................................................... 3 1.1 Eligible area ................................................................................................................... 4 1.2 Project background ....................................................................................................... 5 1.3 Project’s goals ............................................................................................................... 6 1.4 Expected results of the project .................................................................................... 7 2. Report of the event ........................................................................................................... 9 2.1 Publication of an open invitation for the event ................................................................ 9 2.1 Characteristics of Evaluation Criteria ............................................................................... 11 2.2 Selection Criteria ............................................................................................................... 14 3. Winners of competition .................................................................................................. 17 3.1 A few -
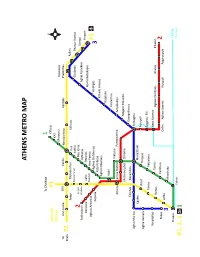
Athens Metro Map
ATHENS METRO MAP To Chalkida 1 Suburban P1 Kifissia Railway ΚΑΤ Maroussi Doukissis To Ano Liosia SΚΑ Iraklio Neratziotissa Pentelis Plakentias Kiato P2 Zefyri Metamorfosi Irini Kifissias Pallini Kato Acharne Iraklio Chalandri Peania- Kantza 2 Pyrgos Nea Ionia Koropi Anthoupoli Vasilissis Pefkakia Aghia Paraskevi Airport Peristeri Aghioi Perissos Nomismatokopio Anargyroi Aghios Antonios Ano Patisia Aghios Eleftherios Holargos Sepolia P3 Kato Patissia 3 Ethniki Amyna Aghios Nikolaos Katehaki Attiki Panormou Viktoria Athens Larissa Station Panepistimio Ampelokipi Metaxourghio Omonia Megaro Mousikis Eleonas Keramikos Evangelismos Aghia Marina Syntagma Egaleo Monastiraki Rouf Acropoli Aghia Varvara Thisseio Syggrou- FIX Tavros Petralona Korydallos Neos Kosmos Rentis Tavros Aghios Dimitrios Alimos Elliniko Nikea Kallithea 2 Lefka Dafni Aghios Ioannis Ilioupoli Argyroupoli 3 Moschato Piraeus Tram Faliro to Voula P1, 2, 3 1 THESSALONIKI BUS LINES N.S. MAKEDONIAAIRPORT STATHMOS LAOGRAFIKO MOUSEIO ARRIVALS AGORA BALTA KAMARA FALIRO KRIKELA ZOGRAFOU KOLOMVOU ΑG. FOTINI ASTYNOMIA KTEL BUSES MAKEDONIAAIRPORT THESSALONIKIS PERIF. ENOTITA DEPARTURES PAN. MAKEDONIAS MEGARO25is MOUSIKIS MARTIOU PLATEIA ARISTOTELOUS DIMARCHIAKO MEGARO LINE 01Χ COSMOS KTEL BUSES MICHANOURGION.S. OSE STATHMOS AGHION PANTON KOLOMVOU ALKAZAR KAMARA AHEPA IPPOKRATIO 25is MARTIOU AGHIAS ANASTASIASMEDITERRANEAN LINE 45 ANTIGONIDON ΚΤΕL CHALKIDIKIS PLATEIA ARISTOTELOUS OTHER BASIC BUS LINES NUMBER TERMINALS AREAS COVERED NUMBER TERMINALS AREAS COVERED EFKARPIA T.S.- SKLAVENITIS- 01 PAPAGEORGIOU HOSP.- EFEDROUPOLI- POLICHNI- PAVLOU MELA - EVOSMOS- EL. KORDELIO- DENDROPOTAMOS- MENEMENI 19 EL. KORDELIO- N.S. STATHMOS ELEFTHERIO KORDELIO- EVOSMOS- DENDROPOTAMOS- MENEMENI- Ν.N. RAILWAY STATION KTEL 02 Α.S. ΙΚΕΑ- KTEL MAKEDONIA NEA KRINI- FINIKAS- DEPO/ KARAMPOURNAKI- VOULGARI- EGNATIA- VARDARIS 21 EVOSMOS- ARISTOTELOUS ΕVOSMOS- AMPELOKIPI- VARDARIS- DIKASTIRION SQ. NEA KRINI- ARERTSOU- KALAMARIA- KARAMPOURNAKI- V. -

State of Play Analyses for Thessaloniki, Greece
State of play analyses for Thessaloniki, Greece Contents Socio-economic characterization of the region ................................................................ 2 Hydrological data .................................................................................................................... 20 Regulatory and institutional framework ......................................................................... 23 Legal framework ...................................................................................................................... 25 Applicable regulations ........................................................................................................... 1 Administrative requirements ................................................................................................ 6 Monitoring and control requirements .................................................................................. 7 Identification of key actors .............................................................................................. 14 Existing situation of wastewater treatment and agriculture .......................................... 23 Characterization of wastewater treatment sector: ................................................................ 23 Characterization of agricultural sector: .................................................................................. 27 Existing related initiatives ................................................................................................ 38 Discussions -

Benefits on Public Health from Transport-Related Greenhouse Gas
Science of the Total Environment 579 (2017) 1427–1438 Contents lists available at ScienceDirect Science of the Total Environment journal homepage: www.elsevier.com/locate/scitotenv Benefits on public health from transport-related greenhouse gas mitigation policies in Southeastern European cities D.A. Sarigiannis a,b,c,⁎, P. Kontoroupis a,b,S.Nikolakia,b, A. Gotti a,c, D. Chapizanis a,S.Karakitsiosa,c a Environmental Engineering Laboratory, Department of Chemical Engineering, Aristotle University of Thessaloniki, GR-54124 Thessaloniki, Greece b Chemical Process Engineering Research Institute, Centre for Research and Technology Hellas, Thermi, Thessaloniki, GR-57001, Greece c Institute for Advanced Study (IUSS), Piazza della Vittoria 15, 27100 Pavia, Italy HIGHLIGHTS GRAPHICAL ABSTRACT • Underground rail reduces health impact attributed to PMx in cities. • Changes in traffic composition reduce health impacts attributed to NO2 and C6H6. • Monetary savings from PM10 and PM2.5 exposure correspond to 60 and 49 mil- lion Euro. • Monetary savings from NO2 and C6H6 exposure correspond to 41 and 1 mil- lion Euro. • 3–4% reduction in mortality and mor- bidity from green transport policies. article info abstract Article history: Climate change is a major environmental threat of our time. Cities have a significant impact on greenhouse gas Received 16 August 2016 emissions as most of the traffic, industry, commerce and more than 50% of world population is situated in Received in revised form 19 November 2016 urban areas. Southern Europe is a region that faces financial turmoil, enhanced migratory fluxes and climate Accepted 20 November 2016 change pressure. The case study of Thessaloniki is presented, one of the only two cities in Greece with established Available online 3 December 2016 climate change action plans.