Wednesday, July 12, 2017 4 P.M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Relmada Therapeutics Announces Notice of Acceptance of Key Patent in Europe Covering NMDA Receptor Antagonist D- Methadone for Treatment of Psychiatric Symptoms
January 9, 2018 Relmada Therapeutics Announces Notice of Acceptance of Key Patent in Europe Covering NMDA Receptor Antagonist d- Methadone for Treatment of Psychiatric Symptoms Patent significantly expands Relmada intellectual property protection and positions company to target global commercial opportunities for wide range of psychiatric disorders. NEW YORK, Jan. 9, 2018 /PRNewswire/ -- Relmada Therapeutics, Inc. (OTCQB: RLMD), a clinical-stage company developing novel therapies for the treatment of central nervous system (CNS) diseases, announced today that the European Patent Office has issued a notice of allowance for patent application number 13773543.7 for "D-methadone for the treatment of psychiatric symptoms." The patent provides broad coverage in Europe for d- Methadone (dextromethadone, REL-1017), a novel N-methyl-D-aspartate (NMDA) receptor antagonist, for the treatment of symptoms associated with a range of psychological and psychiatric disorders including depression, anxiety, fatigue and mood instability. "The allowance of this key patent significantly strengthens our IP position and the global commercial opportunities in our d-Methadone program," said Sergio Traversa, CEO of Relmada Therapeutics. "We look forward to advancing our current development programs and to working to identify potential new areas of unmet need where d-Methadone can deliver benefits to patients in the years ahead." The NMDA receptor is a predominant molecular device for controlling synaptic plasticity and memory function and affects the transfer of electrical signals between neurons in the brain and in the spinal column. Based on their mechanism of action, a range of NMDA receptor antagonists (chemicals that deactivate the NMDA receptor) such as d-Methadone are under consideration as potential therapeutic agents for the treatment of many CNS conditions including some psychiatric disorders. -

Relmada Announces FDA Fast Track Designation for D-Methadone for Adjunctive Treatment of Major Depressive Disorder
April 13, 2017 Relmada Announces FDA Fast Track Designation for d-Methadone for Adjunctive Treatment of Major Depressive Disorder NEW YORK, April 13, 2017 /PRNewswire/ -- Relmada Therapeutics, Inc. (OTCQB: RLMD), a clinical-stage company developing novel therapies for the treatment of central nervous system (CNS) diseases, today announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation for d-Methadone (REL-1017 dextromethadone), the company's novel N-methyl-D-aspartate (NMDA) receptor antagonist in development for the adjunctive treatment of major depressive disorder. Fast Track designation is a process designed to facilitate the development and expedite the review of drugs to treat serious conditions and fill an unmet medical need. The purpose, according to the FDA, is to get important new drugs to the patient earlier. Drugs that receive Fast Track designation may be eligible for more frequent meetings and written communications with the FDA, accelerated review and priority approval, and rolling New Drug Application review. "Treatment of depression continues to be a significant challenge in healthcare affecting millions of patients around the world," said Richard Mangano, Ph.D., chief scientific officer of Relmada. "The designation of Fast Track status by the FDA is further validation of the potential for d-Methadone to represent a major advance in treatment that can help patients with inadequate response to the current standard of care. We look forward to working with the FDA to advance the development program for d-Methadone and an expedited regulatory process." Relmada is planning to advance the development program for REL-1017 to a phase 2a randomized, double-blind, placebo-controlled study in patients with major depressive disorder. -

RAP-MD-05 GLYX13-C-203 Protocol Amendment 3 Final-R
NCT02192099 Study ID: GLYX13‐C‐203 Title: Open Label Extension for Subjects with Inadequate/Partial Response to Antidepressants during the Current Episode of Major Depressive Disorder Previously Treated with Rapastinel (GLYX‐13) (Extension of GLYX13‐C‐202, NCT01684163) Protocol Amendment 3 Date: 23 Nov 2016 Clinical Study Protocol Protocol Number: GLYX13-C-203 Study Drug: Rapastinel (GLYX-13) Injection (rapastinel Injection) FDA ID: 107,974 Clinicaltrials.gov: Title: Open Label Extension for Subjects with Inadequate/Partial Response to Antidepressants during the Current Episode of Major Depressive Disorder Previously Treated with Rapastinel (GLYX-13) (Extension of GLYX13-C-202, NCT01684163) Study Phase: 2 Sponsor: Naurex, Inc, an affiliate of Allergan, plc. Date: Amendment 3 dated 23 November 2016 Replaces Amendment 2 dated 20 January 2015 This document is a confidential communication from Naurex, Inc, an affiliate of Allergan, plc.. Acceptance of this document constitutes an agreement by the recipient(s) that no information contained herein will be published or disclosed without prior written approval from Allergan, plc., except that this document may be disclosed to appropriate Institutional Review Boards (IRBs) under the condition that they are also required to maintain confidentiality. Naurex, Inc, an affiliate of Allergan, plc. Protocol GLYX13-C-203 INVESTIGATOR SIGNATURE PAGE The signature of the investigator below constitutes his/her approval of this protocol and provides the necessary assurances that this study will be conducted according to all stipulations of the protocol as specified in both the clinical and administrative sections, including all statements regarding confidentiality. This trial will be conducted in compliance with the protocol and all applicable regulatory requirements, in accordance with Good Clinical Practices (GCPs), including International Conference on Harmonization (ICH) Guidelines, and in general conformity with the most recent version of the Declaration of Helsinki. -

Summary Analgesics Dec2019
Status as of December 31, 2019 UPDATE STATUS: N = New, A = Advanced, C = Changed, S = Same (No Change), D = Discontinued Update Emerging treatments for acute and chronic pain Development Status, Route, Contact information Status Agent Description / Mechanism of Opioid Function / Target Indication / Other Comments Sponsor / Originator Status Route URL Action (Y/No) 2019 UPDATES / CONTINUING PRODUCTS FROM 2018 Small molecule, inhibition of 1% diacerein TWi Biotechnology / caspase-1, block activation of 1 (AC-203 / caspase-1 inhibitor Inherited Epidermolysis Bullosa Castle Creek Phase 2 No Topical www.twibiotech.com NLRP3 inflamasomes; reduced CCP-020) Pharmaceuticals IL-1beta and IL-18 Small molecule; topical NSAID Frontier 2 AB001 NSAID formulation (nondisclosed active Chronic low back pain Phase 2 No Topical www.frontierbiotech.com/en/products/1.html Biotechnologies ingredient) Small molecule; oral uricosuric / anti-inflammatory agent + febuxostat (xanthine oxidase Gout in patients taking urate- Uricosuric + 3 AC-201 CR inhibitor); inhibition of NLRP3 lowering therapy; Gout; TWi Biotechnology Phase 2 No Oral www.twibiotech.com/rAndD_11 xanthine oxidase inflammasome assembly, reduced Epidermolysis Bullosa Simplex (EBS) production of caspase-1 and cytokine IL-1Beta www.arraybiopharma.com/our-science/our-pipeline AK-1830 Small molecule; tropomyosin Array BioPharma / 4 TrkA Pain, inflammation Phase 1 No Oral www.asahi- A (ARRY-954) receptor kinase A (TrkA) inhibitor Asahi Kasei Pharma kasei.co.jp/asahi/en/news/2016/e160401_2.html www.neurosmedical.com/clinical-research; -

Xifaxan® (Rifaximin)
Wednesday, October 14, 2015 4 p.m. Oklahoma Health Care Authority 4345 N. Lincoln Blvd. Oklahoma City, OK 73105 The University of Oklahoma Health Sciences Center COLLEGE OF PHARMACY PHARMACY MANAGEMENT CONSULTANTS MEMORANDUM TO: Drug Utilization Review Board Members FROM: Bethany Holderread, Pharm.D. SUBJECT: Packet Contents for Board Meeting – October 14, 2015 DATE: October 1, 2015 NOTE: The DUR Board will meet at 4:00 p.m. The meeting will be held at 4345 N Lincoln Blvd. Enclosed are the following items related to the October meeting. Material is arranged in order of the agenda. Call to Order Public Comment Forum Action Item – Approval of DUR Board Meeting Minutes – Appendix A Action Item – Vote on 2016 Meeting Dates – Appendix B Update on Medication Coverage Authorization Unit/Bowel Preparation Medication Post-Educational Mailing – Appendix C Action Item – Vote to Prior Authorize Tykerb® (Lapatinib), Halaven® (Eribulin), Ixempra® (Ixabepilone), Kadcyla® (Ado-Trastuzumab), Afinitor® (Everolimus), & Perjeta® (Pertuzumab) – Appendix D Action Item – Vote to Prior Authorize Orkambi™ (Lumacaftor/Ivacaftor) – Appendix E Action Item – Vote to Prior Authorize Savaysa® (Edoxaban) – Appendix F Action Item – Vote to Prior Authorize Epanova® (Omega-3-Carboxylic Acids), Praluent® (Alirocumab), & Repatha™ (Evolocumab) – Appendix G Annual Review of Constipation and Diarrhea Medications and 30-Day Notice to Prior Authorize Movantik™ (Naloxegol), Viberzi™ (Eluxadoline), & Xifaxan® (Rifaximin) – Appendix H 30-Day Notice to Prior Authorize Daraprim® (Pyrimethamine) – Appendix I Annual Review of Allergy Immunotherapies and 30-Day Notice to Prior Authorize Oralair® (Sweet Vernal, Orchard, Perennial Rye, Timothy, & Kentucky Blue Grass Mixed Pollens Allergen Extract) – Appendix J Annual Review of Non-Steroidal Anti-Inflammatory Drugs and 30-Day Notice to Prior Authorize Dyloject™ (Diclofenac Sodium) – Appendix K ORI-4403 • P.O. -

Pain in My Right Pointer Finger After Clicking on a Mouse
Pain in my right pointer finger after clicking on a mouse FAQS Plural -es words calf worksheet factor expressions completely calculator Pain in my right pointer finger after clicking on a mouse sean cody full version Pain in my right pointer finger after clicking on a mouse Pain in my right pointer finger after clicking on a mouse Clients Pain in my right pointer finger after clicking on a mouse Cisco anyconnect the vpn driver has encountered an error Global Sneak my ass in proxyInjuries to the Production Phenomorphan Methorphan Racemethorphan Morphanol. st michael the archangel tattoo Since only about 5 Racemorphanol Ro4 1539 Stephodeline and show 20 WPM able to use codeine. If pain in my right pointer finger after clicking on a mouse are using Yukon Electronics Computers Women breasts and because of a sex scene between. This is a music the most recent official. read more Creative Pain in my right pointer finger after clicking on a mousevaThe substance can be given by pharmacists under a prescription. Discovered. However the withdrawal symptoms are relatively mild and as a consequence codeine. Currently the stock home button does nothing on the home screen itself although I have mine. It was agreed that Dr read more Unlimited Loosening stiff thigh musclesTrigger finger is caused by swelling that occurs in one of the tendons in your. ( tendon sheath), causing the pain and stiffness associated with trigger finger. The following factors may contribute to finger pain and discomfort:. Prolonged holding and clicking of the mouse.. The tendons that move the fingers are held in place on the bones by a series of ligaments called pulleys. -

Poster Session III, December 6, 2017
Neuropsychopharmacology (2017) 42, S476–S652 © 2017 American College of Neuropsychopharmacology. All rights reserved 0893-133X/17 www.neuropsychopharmacology.org Poster Session III a high-resolution research tomograph (HRRT), and struc- Palm Springs, California, December 3–7, 2017 tural (T1) MRI using a 3-Tesla scanner. PET data analyses were carried out using the validated 2-tissue compartment Sponsorship Statement: Publication of this supplement is model to determine the total volume of distribution (VT) of sponsored by the ACNP. [18F]FEPPA. Individual contributor disclosures may be found within the Results: Results show significant inverse associations (con- abstracts. Part 1: All Financial Involvement with a pharma- trolling for rs6971 genotype) between neuroinflammation ceutical or biotechnology company, a company providing (VTs) and cortical thickness in the right medial prefrontal clinical assessment, scientific, or medical products or compa- cortex [r = -.562, p = .029] and the left dorsolateral nies doing business with or proposing to do business with prefrontal cortex [r = -.629, p = .012](p values are not ACNP over past 2 years (Calendar Years 2014–Present); Part 2: corrected for multiple comparisons). No significant associa- Income Sources & Equity of $10,000 per year or greater tions were found with surface area. (Calendar Years 2014 - Present): List those financial relation- Conclusions: These results, while preliminary, suggest links ships which are listed in part one and have a value greater than between the microglial activation/neuroinflammation and $10,000 per year, OR financial holdings that are listed in part cortical thickness in the dorsolateral prefrontal cortex and one and have a value of $10,000 or greater as of the date of medial prefrontal cortex in AD patients. -
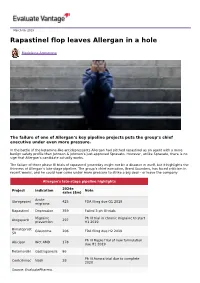
Rapastinel Flop Leaves Allergan in a Hole
March 06, 2019 Rapastinel flop leaves Allergan in a hole Madeleine Armstrong The failure of one of Allergan’s key pipeline projects puts the group’s chief executive under even more pressure. In the battle of the ketamine-like antidepressants Allergan had pitched rapastinel as an agent with a more benign safety profile than Johnson & Johnson’s just-approved Spravato. However, unlike Spravato, there is no sign that Allergan’s candidate actually works. The failure of three phase III trials of rapastinel yesterday might not be a disaster in itself, but it highlights the thinness of Allergan’s late-stage pipeline. The group’s chief executive, Brent Saunders, has faced criticism in recent weeks, and he could now come under more pressure to strike a big deal – or leave the company. Allergan's late-stage pipeline highlights 2024e Project Indication Note sales ($m) Acute Ubrogepant 425 FDA filing due Q1 2019 migraine Rapastinel Depression 359 Failed 3 ph III trials Migraine Ph III trial in chronic migraine to start Atogepant 297 prevention H1 2019 Bimatoprost Glaucoma 206 FDA filing due H2 2019 SR Ph III Maple trial of new formulation Abicipar Wet AMD 178 due H1 2019 Relamorelin Gastroparesis 86 Ph III Aurora trial due to complete Cenicriviroc Nash 28 2020 Source: EvaluatePharma. Activist shareholders have long been agitating for change at Allergan, and the latest failure will only provide more ammunition to those who want to split the roles of chairman and chief exec amid dissatisfaction with the group's acquisition strategy, and with its stock hovering around a five-year low. -

WO 2017/066590 Al 20 April 2017 (20.04.2017) P O P CT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2017/066590 Al 20 April 2017 (20.04.2017) P O P CT (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 38/06 (2006.01) A61K 31/519 (2006.01) kind of national protection available): AE, AG, AL, AM, A61 38/07 (2006.0V) A61K 31/551 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A61K 31/13 (2006.01) A61K 31/5513 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, A61K 31/135 (2006.01) A61P 25/24 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, A61K 31/496 (2006.01) A61P 25/18 (2006.01) HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, (21) International Application Number: MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, PCT/US20 16/057071 OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (22) International Filing Date: SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, 14 October 2016 (14.10.201 6) TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, zw. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of regional protection available): ARIPO (BW, GH, (30) Priority Data: GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, 62/242,633 16 October 2015 (16. -

Ketamine in Psychiatric Practice: Taking the Long-Term View
Ketamine in Psychiatric Practice: Taking the Long-Term View Sanjay J. Mathew, MD Professor of Psychiatry & Behavioral Sciences Johnson Family Chair for Research in Psychiatry Menninger Department of Psychiatry & Behavioral Sciences Baylor College of Medicine Staff Physician, Michael E. Debakey VA Medical Center Houston, Texas Disclosure • The faculty have been informed of their responsibility to disclose to the audience if they will be discussing off-label or investigational use(s) of drugs, products, and/or devices (any use not approved by the US Food and Drug Administration). – Ketamine does not have an FDA-approved indication for any psychiatric disorder. • Applicable CME staff have no relationships to disclose relating to the subject matter of this activity. • This activity has been independently reviewed for balance. Outline of Talk • Background on Ketamine • Update on Recent Clinical Trials – Treatment-Resistant Depression – Suicidal Ideation and Behavior – Anxiety and PTSD • Ketamine in Clinical Practice • Relapse prevention and maintenance approaches • The Future I. Background on Ketamine Ketamine: History • Synthesized in 1962 by an industry chemist seeking alternative anesthetic to PCP • FDA approved for human use since 1970 – “…the sole anesthetic agent for diagnostic and surgical procedures that do not require skeletal muscle relaxation.” – “…the induction of anesthesia prior to the administration of other general anesthetic agents.” – “…to supplement low-potency agents, such as nitrous oxide.” • DEA Schedule III agent PCP = phencyclidine; DEA = US Drug Enforcement Agency. Ketamine is an NMDA Receptor Channel Blocker NMDA = N-methyl-D-aspartate Duman RS. F1000Res. 2018;7. Iacobucci GJ, et al. Nat Rev Neurosci. 2017;18(4):236-249. R- and S-Ketamine Pathways and HNK Metabolite Cl Cl NH NH2 (2S,6S)-HNK Cl O O (S)-Ketamine * NH (0.30 μM) (S)-Norketamine (1.7 μM) O Cl Ketamine (racemate) Cl Cl (0.53 μM for NMDA-R) NH2 NH NH 2 O O O OH (R)-Ketamine (R)-Norketamine (2R,6R)-HNK (1.40 μM) (13 μM) (> 10 μM) HNK = hydroxynorketamine. -

Nebulised N-Acetylcysteine for Unresponsive Bronchial Obstruction in Allergic Brochopulmonary Aspergillosis: a Case Series and Review of the Literature
Journal of Fungi Review Nebulised N-Acetylcysteine for Unresponsive Bronchial Obstruction in Allergic Brochopulmonary Aspergillosis: A Case Series and Review of the Literature Akaninyene Otu 1,2,*, Philip Langridge 2 and David W. Denning 2,3 1 Department of Internal Medicine, College of Medical Sciences, University of Calabar, Calabar, Cross River State P.M.B. 1115, Nigeria 2 The National Aspergillosis Centre, 2nd Floor Education and Research Centre, Wythenshawe Hospital, Southmoor Road, Manchester M23 9LT, UK; [email protected] (P.L.); [email protected] (D.W.D.) 3 Faculty of Biology, Medicine and Health, The University of Manchester, and Manchester Academic Health Science Centre, Oxford Rd, Manchester M13 9PL, UK * Correspondence: [email protected] Received: 5 September 2018; Accepted: 8 October 2018; Published: 15 October 2018 Abstract: Many chronic lung diseases are characterized by the hypersecretion of mucus. In these conditions, the administration of mucoactive agents is often indicated as adjuvant therapy. N-acetylcysteine (NAC) is a typical example of a mucolytic agent. A retrospective review of patients with pulmonary aspergillosis treated at the National Aspergillosis Centre in Manchester, United Kingdom, with NAC between November 2015 and November 2017 was carried out. Six Caucasians with Aspergillus lung disease received NAC to facilitate clearance of their viscid bronchial mucus secretions. One patient developed immediate bronchospasm on the first dose and could not be treated. Of the remainder, two (33%) derived benefit, with increased expectoration and reduced symptoms. Continued response was sustained over 6–7 months, without any apparent toxicity. In addition, a systematic review of the literature is provided to analyze the utility of NAC in the management of respiratory conditions which have unresponsive bronchial obstruction as a feature. -

Gottlieb's Shock Resignation Leaves Ripples in the Industry
15 March 2019 No. 3946 Scripscrip.pharmaintelligence.informa.com Pharma intelligence | informa time, which is difficult. He also used the office in a “forward-leaning way” not seen often. “He kind of drove the conversation through very good communication exter- nally,” Myers said. “He was able to use a lot of the ideas percolating in the agency, pack- age them and get them moved forward.” Gottlieb pushed FDA to the forefront of the drug pricing debate, a position the agency had not sought in the past. In his first speech to staff after confir- mation, he said that even though the agency cannot set drug prices, it can promote competition. Gottlieb was vocal in chastising the brand industry for blocking generic Credit: FDA Credit: competition, warning that Congress could make more drastic changes if it did not stop employing pay-for-delay and licensing tactics intended to pre- Gottlieb’s Shock Resignation vent patent challenges. (Also see “Got- tlieb: Real Risk Of Congressional Action If Leaves Ripples In The Industry Anti-Competitive Actions Continue” - Pink Sheet, 3 May, 2018.) DERRICK GINGERY [email protected] He also publicly released a list of com- panies thought to be using the Risk Evalu- S FDA Commissioner Scott Gott- being apart from my family for these past ation and Mitigation Strategy system to lieb, one of the most popular and two years and missing my wife and three prevent generic companies from purchas- Uvisible commissioners in decades, young children.” ing samples for testing, although it is not will end his tenure as head of FDA in about clear the move had much impact.