1 Perylene-Based Non-Covalent Functionalization of 2D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mild Synthesis of Perylene Tetracarboxylic Monoanhydrides with Potential Applications in Organic Optoelectronics
City University of New York (CUNY) CUNY Academic Works All Dissertations, Theses, and Capstone Projects Dissertations, Theses, and Capstone Projects 5-2019 Mild Synthesis of Perylene Tetracarboxylic Monoanhydrides with Potential Applications in Organic Optoelectronics Xizhe Zhao The Graduate Center, City University of New York How does access to this work benefit ou?y Let us know! More information about this work at: https://academicworks.cuny.edu/gc_etds/3258 Discover additional works at: https://academicworks.cuny.edu This work is made publicly available by the City University of New York (CUNY). Contact: [email protected] Mild Synthesis of Perylene Tetracarboxylic Monoanhydrides with Potential Applications in Organic Optoelectronics By Xizhe Zhao A dissertation submitted to the Graduate Faculty in Chemistry in partial fulfillment of the requirements for the degree of Doctor of Philosophy The City University of New York. 2019 © 2019 Xizhe Zhao All Rights Reserved ii Mild Synthesis of Perylene Tetracarboxylic Monoanhydrides with Potential Applications in Organic Optoelectronics by Xizhe Zhao This manuscript has been read and accepted for the Graduate Faculty in Chemistry in satisfaction of the dissertation requirement for the degree of Doctor of Philosophy. 04/29/19 Prof. Shi Jin _________________________ __________________________________________________ Date Chair of Examining Committee 04/29/19 Prof. Brian R. Gibney _________________________ __________________________________________________ Date Executive Officer Supervisory Committee: Prof. Krishnaswami Raja Prof. Sanjai Kumar Pathak THE CITY UNIVERSITY OF NEW YORK iii ABSTRACT Mild Synthesis of Perylene Tetracarboxylic Monoanhydrides with Potential Applications in Organic Optoelectronics By Xizhe Zhao Advisor: Professor Shi Jin Perylene tetracarboxylic derivatives are considered good n-type semi-conductors. In past decades, there has been extensive study on their synthesis and electronic properties. -

French Names Noeline Bridge
names collated:Chinese personal names and 100 surnames.qxd 29/09/2006 13:00 Page 8 The hundred surnames Pinyin Hanzi (simplified) Wade Giles Other forms Well-known names Pinyin Hanzi (simplified) Wade Giles Other forms Well-known names Zang Tsang Zang Lin Zhu Chu Gee Zhu Yuanzhang, Zhu Xi Zeng Tseng Tsang, Zeng Cai, Zeng Gong Zhu Chu Zhu Danian Dong, Zhu Chu Zhu Zhishan, Zhu Weihao Jeng Zhu Chu Zhu jin, Zhu Sheng Zha Cha Zha Yihuang, Zhuang Chuang Zhuang Zhou, Zhuang Zi Zha Shenxing Zhuansun Chuansun Zhuansun Shi Zhai Chai Zhai Jin, Zhai Shan Zhuge Chuko Zhuge Liang, Zhan Chan Zhan Ruoshui Zhuge Kongming Zhan Chan Chaim Zhan Xiyuan Zhuo Cho Zhuo Mao Zhang Chang Zhang Yuxi Zi Tzu Zi Rudao Zhang Chang Cheung, Zhang Heng, Ziche Tzuch’e Ziche Zhongxing Chiang Zhang Chunqiao Zong Tsung Tsung, Zong Xihua, Zhang Chang Zhang Shengyi, Dung Zong Yuanding Zhang Xuecheng Zongzheng Tsungcheng Zongzheng Zhensun Zhangsun Changsun Zhangsun Wuji Zou Tsou Zou Yang, Zou Liang, Zhao Chao Chew, Zhao Kuangyin, Zou Yan Chieu, Zhao Mingcheng Zu Tsu Zu Chongzhi Chiu Zuo Tso Zuo Si Zhen Chen Zhen Hui, Zhen Yong Zuoqiu Tsoch’iu Zuoqiu Ming Zheng Cheng Cheng, Zheng Qiao, Zheng He, Chung Zheng Banqiao The hundred surnames is one of the most popular reference Zhi Chih Zhi Dake, Zhi Shucai sources for the Han surnames. It was originally compiled by an Zhong Chung Zhong Heqing unknown author in the 10th century and later recompiled many Zhong Chung Zhong Shensi times. The current widely used version includes 503 surnames. Zhong Chung Zhong Sicheng, Zhong Xing The Pinyin index of the 503 Chinese surnames provides an access Zhongli Chungli Zhongli Zi to this great work for Western people. -
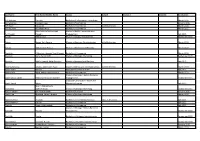
Last Name First Name/Middle Name Course Award Course 2 Award 2 Graduation
Last Name First Name/Middle Name Course Award Course 2 Award 2 Graduation A/L Krishnan Thiinash Bachelor of Information Technology March 2015 A/L Selvaraju Theeban Raju Bachelor of Commerce January 2015 A/P Balan Durgarani Bachelor of Commerce with Distinction March 2015 A/P Rajaram Koushalya Priya Bachelor of Commerce March 2015 Hiba Mohsin Mohammed Master of Health Leadership and Aal-Yaseen Hussein Management July 2015 Aamer Muhammad Master of Quality Management September 2015 Abbas Hanaa Safy Seyam Master of Business Administration with Distinction March 2015 Abbasi Muhammad Hamza Master of International Business March 2015 Abdallah AlMustafa Hussein Saad Elsayed Bachelor of Commerce March 2015 Abdallah Asma Samir Lutfi Master of Strategic Marketing September 2015 Abdallah Moh'd Jawdat Abdel Rahman Master of International Business July 2015 AbdelAaty Mosa Amany Abdelkader Saad Master of Media and Communications with Distinction March 2015 Abdel-Karim Mervat Graduate Diploma in TESOL July 2015 Abdelmalik Mark Maher Abdelmesseh Bachelor of Commerce March 2015 Master of Strategic Human Resource Abdelrahman Abdo Mohammed Talat Abdelziz Management September 2015 Graduate Certificate in Health and Abdel-Sayed Mario Physical Education July 2015 Sherif Ahmed Fathy AbdRabou Abdelmohsen Master of Strategic Marketing September 2015 Abdul Hakeem Siti Fatimah Binte Bachelor of Science January 2015 Abdul Haq Shaddad Yousef Ibrahim Master of Strategic Marketing March 2015 Abdul Rahman Al Jabier Bachelor of Engineering Honours Class II, Division 1 -

Bo-Qing Xu Biography
Bo-Qing Xu_Biography Bo-Qing Xu is a Changjiang Scholar Professor of Chemistry and serves as the Director at the Institute of Physical Chemistry of Tsinghua University. He completed his doctorate in physical chemistry of heterogeneous catalysis at Dalian Institute of Chemical Physics (DICP, Chinese Academy of Sciences) in 1988, as a jointly educated Ph.D. of DICP and Hokkaido University of Japan. He then worked as a research associate at DICP. In December 1991, he joined the faculty of the School of Chemical Engineering at Dalian University of Technology (DUT) and became a full Professor in late 1992. From March 1995 to April 1998, he worked as a visiting scientist fellow at the Catalysis Center in Northwestern University (USA) and at the School of Chemical Engineering in Georgia Institute of Technology. He joined Tsinghua University in May 1998 and had been as a short-term visiting professor at UC Berkeley (2002) and Hong Kong Baptist University (2003). He has served as the vice-president of the Chinese Catalysis Society in 2012-2017, associated editor of ACS Catalysis since February 2014, editorial advisory member of Current Catalysis (2011-), Chinese Journal of Catalysis (2001-), Chinese Journal of Fuel Chemistry (2009-) and Chinese Journal of Environmental Science & Technology (2008-). He was an editorial advisory member of Applied Catalysis A-General (2005-2008) and a gust editor of Topics in Catalysis (2003). He was also an International Advisory Board member of the international Acid-Base Catalysis Group (2001-2013). His main research interest is on the physical chemistry aspects of Heterogeneous Catalysis and Nanostructure Materials for sustainable energy and environments. -

Baby Girl Names Registered in 2018
Page 1 of 46 Baby Girl Names Registered in 2018 Frequency Name Frequency Name Frequency Name 8 Aadhya 1 Aayza 1 Adalaide 1 Aadi 1 Abaani 2 Adalee 1 Aaeesha 1 Abagale 1 Adaleia 1 Aafiyah 1 Abaigeal 1 Adaleigh 4 Aahana 1 Abayoo 1 Adalia 1 Aahna 2 Abbey 13 Adaline 1 Aaila 4 Abbie 1 Adallynn 3 Aaima 1 Abbigail 22 Adalyn 3 Aaira 17 Abby 1 Adalynd 1 Aaiza 1 Abbyanna 1 Adalyne 1 Aaliah 1 Abegail 19 Adalynn 1 Aalina 1 Abelaket 1 Adalynne 33 Aaliyah 2 Abella 1 Adan 1 Aaliyah-Jade 2 Abi 1 Adan-Rehman 1 Aalizah 1 Abiageal 1 Adara 1 Aalyiah 1 Abiela 3 Addalyn 1 Aamber 153 Abigail 2 Addalynn 1 Aamilah 1 Abigaille 1 Addalynne 1 Aamina 1 Abigail-Yonas 1 Addeline 1 Aaminah 3 Abigale 2 Addelynn 1 Aanvi 1 Abigayle 3 Addilyn 2 Aanya 1 Abiha 1 Addilynn 1 Aara 1 Abilene 66 Addison 1 Aaradhya 1 Abisha 3 Addisyn 1 Aaral 1 Abisola 1 Addy 1 Aaralyn 1 Abla 9 Addyson 1 Aaralynn 1 Abraj 1 Addyzen-Jerynne 1 Aarao 1 Abree 1 Adea 2 Aaravi 1 Abrianna 1 Adedoyin 1 Aarcy 4 Abrielle 1 Adela 2 Aaria 1 Abrienne 25 Adelaide 2 Aariah 1 Abril 1 Adelaya 1 Aarinya 1 Abrish 5 Adele 1 Aarmi 2 Absalat 1 Adeleine 2 Aarna 1 Abuk 1 Adelena 1 Aarnavi 1 Abyan 2 Adelin 1 Aaro 1 Acacia 5 Adelina 1 Aarohi 1 Acadia 35 Adeline 1 Aarshi 1 Acelee 1 Adéline 2 Aarushi 1 Acelyn 1 Adelita 1 Aarvi 2 Acelynn 1 Adeljine 8 Aarya 1 Aceshana 1 Adelle 2 Aaryahi 1 Achai 21 Adelyn 1 Aashvi 1 Achan 2 Adelyne 1 Aasiyah 1 Achankeng 12 Adelynn 1 Aavani 1 Achel 1 Aderinsola 1 Aaverie 1 Achok 1 Adetoni 4 Aavya 1 Achol 1 Adeyomola 1 Aayana 16 Ada 1 Adhel 2 Aayat 1 Adah 1 Adhvaytha 1 Aayath 1 Adahlia 1 Adilee 1 -

Ideophones in Middle Chinese
KU LEUVEN FACULTY OF ARTS BLIJDE INKOMSTSTRAAT 21 BOX 3301 3000 LEUVEN, BELGIË ! Ideophones in Middle Chinese: A Typological Study of a Tang Dynasty Poetic Corpus Thomas'Van'Hoey' ' Presented(in(fulfilment(of(the(requirements(for(the(degree(of(( Master(of(Arts(in(Linguistics( ( Supervisor:(prof.(dr.(Jean=Christophe(Verstraete((promotor)( ( ( Academic(year(2014=2015 149(431(characters Abstract (English) Ideophones in Middle Chinese: A Typological Study of a Tang Dynasty Poetic Corpus Thomas Van Hoey This M.A. thesis investigates ideophones in Tang dynasty (618-907 AD) Middle Chinese (Sinitic, Sino- Tibetan) from a typological perspective. Ideophones are defined as a set of words that are phonologically and morphologically marked and depict some form of sensory image (Dingemanse 2011b). Middle Chinese has a large body of ideophones, whose domains range from the depiction of sound, movement, visual and other external senses to the depiction of internal senses (cf. Dingemanse 2012a). There is some work on modern variants of Sinitic languages (cf. Mok 2001; Bodomo 2006; de Sousa 2008; de Sousa 2011; Meng 2012; Wu 2014), but so far, there is no encompassing study of ideophones of a stage in the historical development of Sinitic languages. The purpose of this study is to develop a descriptive model for ideophones in Middle Chinese, which is compatible with what we know about them cross-linguistically. The main research question of this study is “what are the phonological, morphological, semantic and syntactic features of ideophones in Middle Chinese?” This question is studied in terms of three parameters, viz. the parameters of form, of meaning and of use. -

Highly Soluble Monoamino-Substituted Perylene Tetracarboxylic Dianhydrides: Synthesis, Optical and Electrochemical Properties
Int. J. Mol. Sci. 2014, 15, 22642-22660; doi:10.3390/ijms151222642 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Article Highly Soluble Monoamino-Substituted Perylene Tetracarboxylic Dianhydrides: Synthesis, Optical and Electrochemical Properties Kew-Yu Chen * and Che-Wei Chang Department of Chemical Engineering, Feng Chia University, Taichung 40724, Taiwan; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +886-4-2451-7250 (ext. 3683); Fax: +886-4-2451-0890. External Editor: Dennis N. Kevill Received: 8 November 2014; in revised form: 21 November 2014 / Accepted: 1 December 2014 / Published: 8 December 2014 Abstract: Three dialkylamino-substituted perylene tetracarboxylic dianhydrides with different n-alkyl chain lengths (n = 6, 12 or 18), 1a–1c, were synthesized under mild conditions in high yields and were characterized by 1H NMR, 13C NMR and high resolution mass spectroscopy. Their optical and electrochemical properties were measured using UV-Vis and emission spectroscopic techniques, as well as cyclic voltammetry (CV). This is the first time that the structures and the properties of monoamino-substituted perylene tetracarboxylic dianhydrides have been reported. These molecules show a deep green color in both solution and the solid state and are soluble in most organic solvents. They all show a unique charge transfer emission in the near-infrared region, and the associated peaks exhibit solvatochromism. The dipole moments of the compounds have been estimated using the Lippert-Mataga equation, and upon excitation, they show slightly larger dipole moment changes than those of corresponding perylene diimides, 2a–2c. -

Polycyclic Aromatic Hydrocarbon Structure Index
United States Department of Commerce Technology Administration National Institute of Standards and Technology NIST Special Publication 922 Polycyclic Aromatic Hydrocarbon Structure Index Lane C. Sander and Stephen A. Wise This publication is available free of charge from: https://doi.org/10.6028/NIST.SP.922e2020 NIST Special Publication 922 Polycyclic Aromatic Hydrocarbon Structure Index Lane C. Sander and Stephen A. Wise Chemical Science and Technology Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899-0001 December 1997 revised August 2020 This publication is available free of charge from: https://doi.org/10.6028/NIST.SP.922e2020 U.S. Department of Commerce Wilbur L. Ross, Jr., Secretary National Institute of Standards and Technology Walter Copan, NIST Director and Under Secretary of Commerce for Standards and Technology Errata for SP 922 (printed document) Corrected entries are listed below 2 1 3 19 Acephenanthrylene CH 9 10 4 201-06-9 16 10 202 1.291 8 5 Cyclopenta[jk]phenanthrene 11.70 7 6 9.067 3.890 10 1 9 2 27208-37-3 226 1.200 33 8 3 Cyclopenta[cd]pyrene CH18 10 7 4 11.52 6 5 Acepyrene Acepyrylene 9.602 3.887 10 9 1 34 Benz[mno]aceanthrylene 203-13-4 CH 226 8 2 18 10 1.222 7 3 Naphtho[1,8,7,6-cdef]fluorene 11.83 6 5 4 9.679 3.884 2 HC2 1 3 41 11 4 1H-Cyclopenta[e]pyrene 109587-09-9 CH19 12 240 10 5 9 8 6 7 11 1 10 2 9 3 52 5H-Benzo[cd]pyrene 191-34-4 CH19 12 240 1.233 8 5 4 7 6 Pyrenindene 11.92 C 9.674 H2 4.207 14 1 13 2 12 3 160 4 Cyclopent[b]indeno[4,5-g]- 72088-81-4 CH24 14 302 10 11 9 phenanthrene 8 5 7 6 2 3 1 4 14 5 Cyclopent[b]indeno[5,6-g]- 72088-82-5 CH 302 161 6 24 14 12 13 phenanthrene 11 10 7 89 Polycyclic Aromatic Hydrocarbon Structure Index Lane C. -

Ying Zhuo, Li Bao, Zhichan Chen, Yuemin Liu, Xinyu Xu, Xia Chai, Peng Zhang, Yong Huang
International Journal of Clinical Acupuncture Volume 15, Number 2, 2006 CONTENTS CLINICAL RESEARCH A Clinical Observation of Treating 32 Cases of Ankylosing Spondylitis by Bo’s Abdominal Acupuncture Ying Zhuo, Li Bao, Zhichan Chen, Yuemin Liu, Xinyu Xu, Xia Chai, Peng Zhang, Yong Huang Clinical Observation of Elongated Needle Treatment of Lumbar Intervertebral Disc Prolapse Xiuli Yuan, Chi Liu, Juan Wang Clinical Observation of Abdominal Cluster-needling For the Treatment of Chronic Adnexitis with Infertility Jianyun Hong, Lei Chen, Yanyan Zhang, Yong Liang Clinical Observation of Treating the Acute Stage of Peripheral Facial Paralysis by Bleeding-cupping SJ 17 Huangtong Li, Jianhua Liu, Jing Chen Clinical Observation of Treating Migraine with Deep Needling at Point Fengchi Weizhe Deng,ZhixinYang The Observation of Curative Effects on Cervical Spondylosis with a Combination of Acupuncture and Tuina Ling Gao, Yannan Liu, Zhaohui Wang MECHANISM RESEARCH Improvement of Gastric Electrical Activities on Patients with Functional Dyspepsia Treated by Acupuncture Danan Sun, Junxian Cao EXPERIENCE OF FAMOUS SPECIALISTS A Brief Introduction to Professor Lai’s Clinical Experience in Treating Cerebral International Journal of Clinical Acupuncture Volume 15, Number 2, 2006 Diseases Yong Huang ANCIENT ACUPUNCTURE LITERATURE RESEARCH Classical Needling Techniques from the Nan Jing (The Classic of Difficult Issues) Wen Jiang, Changzhen Gong LECTURE A Introduction on Reducing Weight by Acupuncture Mohamed Aly el Sherbiny MANIPULATION RESEARCH Comments on the Depth of Needle Insertion and Precautions for Acupuncture Therapy Jue Zhou, Jianling Liu SHORT PAPERS Acupuncture for Acute Asthma Attack Caused by AllergyHaifei Yan Qun Hang, Qingguo Wang MINI-REVIEW Progress in Acupuncture Treatment of Prostatic Hyperplasia Jianwen Wang, Shiqun Liu . -

Polycyclic Aromatic Hydrocarbons (Pahs)
Polycyclic Aromatic Hydrocarbons (PAHs) Factsheet 4th edition Donata Lerda JRC 66955 - 2011 The mission of the JRC-IRMM is to promote a common and reliable European measurement system in support of EU policies. European Commission Joint Research Centre Institute for Reference Materials and Measurements Contact information Address: Retiewseweg 111, 2440 Geel, Belgium E-mail: [email protected] Tel.: +32 (0)14 571 826 Fax: +32 (0)14 571 783 http://irmm.jrc.ec.europa.eu/ http://www.jrc.ec.europa.eu/ Legal Notice Neither the European Commission nor any person acting on behalf of the Commission is responsible for the use which might be made of this publication. Europe Direct is a service to help you find answers to your questions about the European Union Freephone number (*): 00 800 6 7 8 9 10 11 (*) Certain mobile telephone operators do not allow access to 00 800 numbers or these calls may be billed. A great deal of additional information on the European Union is available on the Internet. It can be accessed through the Europa server http://europa.eu/ JRC 66955 © European Union, 2011 Reproduction is authorised provided the source is acknowledged Printed in Belgium Table of contents Chemical structure of PAHs................................................................................................................................. 1 PAHs included in EU legislation.......................................................................................................................... 6 Toxicity of PAHs included in EPA and EU -

Chinese Language and Characters
Chinese Language and Characters Pronunciation of Chinese Words Consonants Pinyin WadeGiles Pronunciation Example: Pinyin(WadeGiles) Aspirated: p p’ pin Pao (P’ao) t t’ tip Tao (T’ao) k k’ kilt Kuan (K’uan) ch ch’ ch in, ch urch Chi (Ch’i) q ch’ ch eek Qi (Ch’i) c ts’ bi ts Cang (Ts’ang) Un- b p bin Bao (Pao) aspirated: d t dip Dao (Tao) g k gilt Guan (Kuan) r j wr en Ren (Jen) sh sh sh ore Shang (Shang) si szu Si (Szu) x hs or sh sh oe Xu (Hsu) z ts or tz bi ds Zang (Tsang) zh ch gin Zhong (Chong) zh j jeep Zhong (Jong) zi tzu Zi (Tzu) Vowels - a a father usually Italian e e ei ght values eh eh broth er yi i mach ine, p in Yi (I) i ih sh ir t Zhi (Chih) o soap u goo se ü über Dipthongs ai light ao lou d ei wei ght ia Will ia m ieh Kor ea ou gr ou p ua swa n ueh do er ui sway Hui (Hui) uo Whoah ! Combinations ian ien Tian (Tien) ui wei Wei gh Shui (Shwei) an and ang bun and b ung en and eng wood en and am ong in and ing sin and s ing ong un and ung u as in l oo k Tong (T’ung) you yu Watts, Alan; Tao The Watercourse Way, Pelican Books, 1976 http://acc6.its.brooklyn.cuny.edu/~phalsall/texts/chinlng1.html Tones 1 2 3 4 ā á ă à ē é ĕ È è Ī ī í ĭ ì ō ó ŏ ò ū ú ŭ ù Pinyin (Wade Giles) Meaning Ai Bā (Pa) Eight, see Numbers Bái (Pai) White, plain, unadorned Băi (Pai) One hundred, see Numbers Bāo Envelop Bāo (Pao) Uterus, afterbirth Bēi Sad, Sorrow, melancholy Bĕn Root, origin (Biao and Ben) see Biao Bi Bi (bei) Bian Bi āo Tip, dart, javelin, (Biao and Ben) see Ben Bin Bin Bing Bu Bu Can Cang Cáng (Ts’ang) Hidden, concealed (see Zang) Cháng Intestine Ch ōng (Ch’ung) Surging Ch ōng (Ch’ung) Rushing Chóu Worry Cóng Follow, accord with Dăn (Tan) Niche or shrine Dăn (Tan) Gall Bladder Dān (Tan) Red Cinnabar Dào (Tao) The Way Dì (Ti) The Earth, i.e. -

Supplemental Comments on EPA's Draft Risk Evaluation for C.I. Pigment Violet 29
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY Supplemental Comments of Safer Chemicals Healthy Families, Earthjustice, Environmental Health Strategy Center, Natural Resources Defense Council and the Undersigned Groups on EPA’s Draft Risk Evaluation for C.I. Pigment Violet 29 under the Amended Toxic Substances Control Act Submitted via Regulations.gov (May 17, 2019) Docket ID EPA-HQ-OPPT-2018-0604 INTRODUCTION AND SUMMARY Safer Chemicals Healthy Families (SCHF), Earthjustice, Environmental Health Strategy Center, Natural Resources Defense Council, and the undersigned groups submit these supplemental comments on the Environmental Protection Agency (EPA) draft risk evaluation for C.I. Pigment Violet 29 (PV29) under section 6(B) of the Toxic SuBstances Control Act (TSCA).1 Our organizations are committed to assuring the safety of chemicals used in our homes, schools and workplaces and in the many products to which our families and children are exposed each day. During the legislative process to amend TSCA, we worked hard to maximize public health protection and to assure that EPA has the necessary authority to evaluate and eliminate the risks of unsafe chemicals. We strongly support a proactive approach to implementing the new law that uses the improved tools that Congress gave EPA to deliver significant health and environmental benefits to the American public. On April 17, 2019,2 EPA reopened the comment period on the PV29 evaluation for 30 days in order to receive feedback on supplemental materials it had added to the PV29 docket. These materials include portions of 24 studies on PV29 that the Agency had initially withheld as Confidential Business Information (CBI), as well as updated systematic review files and backup scoring sheets.