Schneider's Smooth-Fronted Caiman Paleosuchus Trigonatus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Crocodiles and Alligators Capture Production by Species, Fishing Areas
394 Crocodiles and alligators Capture production by species, fishing areas and countries or areas B-73 Crocodiles et alligators Captures par espèces, zones de pêche et pays ou zones Cocodrilos y aligatores Capturas por especies, áreas de pesca y países o áreas Species, Fishing area Espèce, Zone de pêche 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 Especie, Área de pesca no no no no no no no no no no Broad-nosed caiman ...B ...C Caiman latirostris 5,36(01)001,01 IMO 03 Argentina - - - 90 165 215 2 752 1 652 1 125 705 Brazil - - - - - 10 ... 50 - - 03 Fishing area total - - - 90 165 225 2 752 1 702 1 125 705 Species total - - - 90 165 225 2 752 1 702 1 125 705 Spectacled caiman Caïman à lunettes Caimán de anteojos Caiman crocodilus 5,36(01)001,03 CAI 02 Nicaragua 250 6 440 ... ... ... ... ... ... ... 130 Panama 10 10 250 11 700 13 298 14 694 15 850 4 696 2 210 2 752 1 155 02 Fishing area total 260 16 690 11 700 13 298 14 694 15 850 4 696 2 210 2 752 1 285 03 Argentina - - - - - 1 1 291 2 883 6 083 3 556 Bolivia 17 500 ... 28 170 31 018 43 528 36 299 51 330 44 443 49 115 51 618 Brazil 4 619 8 286 1 253 6 048 12 851 7 004 620 673 10 254 ... Colombia 771 456 832 203 704 313 540 579 555 719 612 041 601 958 974 721 673 062 535 394 Guyana 9 880 9 880 5 917 8 222 3 124 3 310 2 301 3 720 16 707 3 355 Paraguay - 9 750 3 793 8 373 4 409 - - - - - Venezuela 24 640 23 655 19 215 20 349 33 942 63 902 58 346 60 864 23 201 15 489 03 Fishing area total 828 095 883 774 762 661 614 589 653 573 722 557 715 846 1 087 304 778 422 609 412 Species total -

Identification Notes &~@~-/~: ~~*~@~,~ 'PTILE
CATEGORY Identification Notes &~@~-/~: ~~*~@~,~ ‘PTILE for wildlife law enforcement ~ C.rnrn.n N.rn./s: Al@~O~, c~~~dil., ~i~.xl, Gharial PROBLEM: Skulls of Crocodilians are often imported as souvenirs. nalch (-”W 4(JI -“by ieeth ??la&ularJy+i9 GUIDE TO PRELIMINARY IDENTIFICATION OF CROCODILL4N SKULLS 1. Nasal bones separated from nasal aperture; mandibular symphysis extends to the 15th tooth. 2. Gavialis gangeticus Nasal bones entering the nasal aperture; mandibular symphysisdoes not extend beyond the8th tooth . Tomistoma schlegelii 2. Nasal bones separated from premaxillary bones; 27 -29maxi11aryteeth,25 -26mandibularteeth Nasal bones in contact with premaxillaq bo Qoco@khs acutus teeth, 18-19 mandibular teeth . Tomiitomaschlegelii 3. Fourth mandibular tooth usually fitting into a notch in the maxilla~, 16-19 maxillary teeth, 14-15 mandibular teeth . .4 Osteolaemus temaspis Fourth mandibular tooth usually fitting into a pit in the maxilla~, 14-20 maxillary teeth, 17-22 mandibular teeth . .5 4. Nasal bones do not divide nasal aperture. .. CrocodylW (12 species) Alligator m&siss@piensh Nasalboncx divide nasal aperture . Osteolaemustetraspk. 5. Nasal bones do not divide nasal aperture. .6 . Paleosuchus mgonatus Bony septum divides nasal aperture . .. Alligator (2 species) 6. Fiveteethinpremaxilla~ bone . .7 . Melanosuchus niger Four teeth in premaxillary bone. ...Paleosuchus (2species) 7. Vomerexposed on the palate . Melanosuchusniger Caiman crocodiles Vomer not exposed on palate . ...”..Caiman (2species) Illustrations from: Moo~ C. C 1921 Me&m, F. 19S1 L-.. Submitted by: Stephen D. Busack, Chief, Morphology Section, National Fish& Wildlife Forensics LabDate submitted 6/3/91 Prepared in cooperation with the National Fkh & Wdlife Forensics Laboratoy, Ashlar@ OR, USA ‘—m More on reverse side>>> IDentMcation Notes CATEGORY: REPTILE for wildlife law enforcement -- Crocodylia II CAmmom Nda Alligator, Crocodile, Caiman, Gharial REFERENCES Medem, F. -

Growth Rates of Paleosuchus Palpebrosus at the Southern Limit of Its Range Author(S): Zilca Campos , William E
Growth Rates of Paleosuchus palpebrosus at the Southern Limit of its Range Author(s): Zilca Campos , William E. Magnusson , and Vanílio Marques Source: Herpetologica, 69(4):405-410. 2013. Published By: The Herpetologists' League DOI: http://dx.doi.org/10.1655/HERPETOLOGICA-D-13-00005 URL: http://www.bioone.org/doi/full/10.1655/HERPETOLOGICA-D-13-00005 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/ terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Herpetologica, 69(4), 2013, 405–410 Ó 2013 by The Herpetologists’ League, Inc. GROWTH RATES OF PALEOSUCHUS PALPEBROSUS AT THE SOUTHERN LIMIT OF ITS RANGE 1,4 2 3 ZILCA CAMPOS ,WILLIAM E. MAGNUSSON , AND VANILIO´ MARQUES 1Laboratorio´ de vida selvagem, Embrapa Pantanal, CP 109, Corumba´, MS, 793200-900, Brazil 2Coordena¸ca˜o de Biodiversidade, Instituto Nacional de Pesquisa da Amazonia,ˆ CP 2223, Manaus, AM, 69080-971, Brazil 3Instituo Chico Mendes de Conserva¸ca˜o de Biodiversidade, CP 100, Cuiaba´, MT, 78055-900, Brazil ABSTRACT: We estimated growth rates of Dwarf Caiman (Paleosuchus palpebrosus) with capture– recapture data from 40 individuals collected over 6 yr in streams surrounding the Brazilian Pantanal, near the southern limit of the species’ distribution. -

(Schneider, 1801) (Crocodylia: Alligatoridae), in the Amazon–Cerrado Transition, Brazil
13 4 91–94 Date 2017 NOTES ON GEOGRAPHIC DISTRIBUTION Check List 13 (4): 91–94 https://doi.org/10.15560/13.4.91 Extension of the geographical distribution of Schneider’s Dwarf Caiman, Paleosuchus trigonatus (Schneider, 1801) (Crocodylia: Alligatoridae), in the Amazon–Cerrado transition, Brazil Zilca Campos,1 Fábio Muniz,2 William E. Magnusson2 1 Embrapa Pantanal, CP 109, 79320-900 Corumbá, MS, Brazil. 2 Instituto Nacional de Pesquisas da Amazônia, CP 2223, 69080-971 Manaus, AM, Brazil. Corresponding author: Zilca Campos, [email protected] Abstract We present new records of occurrence of Schneider’s Dwarf Caiman, Paleosuchus trigonatus and extend its geo- graphical distribution. Eight individuals were caught in the following locations: Sangue River, in the municipality of Campo Novo dos Parecis, Claro River and Marapi River, in the municipality of São José do Rio Claro, and tributaries of the Juruena River, in the state of Mato Grosso, Brazil. These records extend the geographical distribution of the species nearly 500 km south of the limit given in published range maps. Key words New records; conservation; Paleosuchus; Mato Grosso; Brazilian Amazon. Academic editor: Raul F. D. Sales | Received 30 June 2016 | Accepted 10 April 2017 | Published 12 July 2017 Citation: Campos Z, Muniz F, Magnusson WE (2017) Extension of the geographical distribution of Schneider’s Dwarf Caiman, Paleosuchus trigonatus (Schneider, 1801) (Crocodylia: Alligatoridae), in the Amazon-Cerrado transition, Brazil. Check List 13 (4): 91–94. https://doi. org/10.15560/13.4.91 Introduction known geographical distribution of P. trigonatus. These are the first occurrence records of the species in the Cer- The geographical distribution of Schneider’s Dwarf Cai- rado biome although still in the Amazon drainage. -

The Discovery of Cuvier's Dwarf Caiman, Paleosuchus Palpebrosus
Nature Notes 41 The discovery of Cuvier’s Dwarf Caiman, Paleosuchus palpebrosus (Reptilia: Alligatoridae) in Trinidad Trinidad has a rich diversity of reptiles. Murphy (1997) Augustine (UWIZM.2016.35) and a tissue sample for reported 91 species of reptiles including introduced spe- DNA was collected. cies, species of questionable occurrence, and waifs. The Two Paleosuchus palpebrosus were collected at 1997 summary also included a discussion of three species Granville in 2014. These were both very young individu- of crocodilians possibly present on Trinidad. The Specta- als caught in a stream between Austin Coromandel North cled Caiman, Caiman crocodilus is the only crocodilian and Syfo Trace, Granville in southwestern Trinidad (Grid known to have established populations on both Trinidad Reference UTM 20-n 632730m E 1118206m N). One was and Tobago. It can be found in most swamps and water approximately 25cm long, and caught about 2315 h on courses from the southern versant of the Northern Range 22 February 2014. The second was approximately 26.5cm to the south coast (Gerard 1991, Mohammed 2015). Spec- long, caught at 2105 h on 1 March 2014. Finally, a female, ulation over the past century has suggested that two croco- 86.5cm long, was discovered on 11 October 2016 at 2245 h diles, the American Crocodile, Crocodylus acutus and the at the Iros Forest between Chattam North Trace and Point Orinoco Crocodile, C. intermedius may occur as waifs in Coco Trace Ext. (UTN 20-n 635028m E, 1121110m N). Trinidad and Tobago waters. However, the evidence for These are the first reports of this species for Trinidad this is scant and summarized in Murphy (1997). -

Crocodylia, Alligatoridae) After a Half-Century Delay: Bridging the Gap in the Chromosomal Evolution of Reptiles
cells Article Revisiting the Karyotypes of Alligators and Caimans (Crocodylia, Alligatoridae) after a Half-Century Delay: Bridging the Gap in the Chromosomal Evolution of Reptiles Vanessa C. S. Oliveira 1 , Marie Altmanová 2,3 , Patrik F. Viana 4 , Tariq Ezaz 5 , Luiz A. C. Bertollo 1, Petr Ráb 3, Thomas Liehr 6,* , Ahmed Al-Rikabi 6, Eliana Feldberg 4, Terumi Hatanaka 1, Sebastian Scholz 7, Alexander Meurer 8 and Marcelo de Bello Cioffi 1 1 Laboratório de Citogenética de Peixes, Departamento de Genética e Evolução, Universidade Federal de São Carlos, São Carlos 13565-905, Brazil; [email protected] (V.C.S.O.); [email protected] (L.A.C.B.); [email protected] (T.H.); mbcioffi@ufscar.br (M.d.B.C.) 2 Department of Ecology, Faculty of Science, Charles University, 12844 Prague, Czech Republic; [email protected] 3 Laboratory of Fish Genetics, Institute of Animal Physiology and Genetics, Czech Academy of Sciences, 27721 Libˇechov, Czech Republic; [email protected] 4 Laboratório de Genética Animal, Coordenação de Biodiversidade, Instituto Nacional de Pesquisas da Amazônia, Manaus 69083-000, Brazil; [email protected] (P.F.V.); [email protected] (E.F.) 5 Institute for Applied Ecology, Faculty of Science and Technology, University of Canberra, Bruce, ACT 2617, Australia; [email protected] 6 Institute of Human Genetics, Jena University Hospital, Am Klinikum 1, 07747 Jena, Germany; [email protected] Citation: Oliveira, V.C.S.; 7 An der Nachtweide 16, 60433 Frankfurt, Germany; [email protected] Altmanová, M.; Viana, P.F.; Ezaz, T.; 8 Alfred Nobel Strasse 1e, 55411 Bingen am Rhein, Germany; [email protected] Bertollo, L.A.C.; Ráb, P.; Liehr, T.; * Correspondence: [email protected]; Tel.: +49-36-41-939-68-50; Fax: +49-3641-93-96-852 Al-Rikabi, A.; Feldberg, E.; Hatanaka, T.; et al. -

09. Crocodylian Scatology
Hunt et al., eds., 2012, Vertebrate Coprolites. New Mexico Museum of Natural History and Science, Bulletin 57. 65 CROCODYLIAN SCATOLOGY – A LOOK INTO MORPHOLOGY, INTERNAL ARCHITECTURE, INTER- AND INTRASPECIFIC VARIATION AND PREY REMAINS IN EXTANT CROCODYLIAN FECES JESPER MILÀN1,2 1 GeomuseumFaxe/Østsjællands Museum, Østervej 2, DK-4640 Faxe, Denmark, e-mail: [email protected]; 2 Department of Geography and Geology, University of Copenhagen, Øster Voldgade 10, DK-1350 Copenhagen K, Denmark Abstract—Seventeen specimens of fresh scats from 10 species of extant crocodylians from CrocodileZoo in Denmark are compared with regard to morphology, internal architecture, inter- and intraspecific variation and undigested prey remains. Crocodylian feces are typically cylindrical to tapering with rounded terminations, and longitudinal striations in the surface were observed in one specimen. They often have one or two bends of 120- 150°. Internally, they consist of concentric layers of darker clay-like material and a lighter mass containing undigested prey remains. The prey remains (piglets, rats and chickens) comprise relatively well-preserved hair, and partly dissolved feathers with only the rachis left. Even when sieved to a mesh size of 0.122 mm, no remains of bones could be found. Scats from a gharial with a strictly piscivorous diet contained no remains of bones or scales. The diameter of the feces correlates well with the total body length of the animal. This enables a reasonable estimate of the producers’ size from fossil coprolites. The intraspecific variation in morphology within crocodylian feces reflects the full spectrum of observed interspecific variations, making it impossible to distinguish scats of different species from each other. -

Schneider's Smooth-Fronted Caiman Paleosuchus Trigonatus
Schneider’s Smooth-fronted Caiman Paleosuchus trigonatus William E. Magnusson1 and Zilca Campos2 1 Instituto Nacional de Pesquisas da Amazonia/CPEC, CP 478, Manaus, Amazonas 69011-970, Brazil ([email protected]) 2 Embrapa Pantanal, CP 109, Corumbá, MS, Brazil 79320-900 ([email protected]) Common Names: Smooth-fronted caiman, Schneider’s 2009 IUCN Red List: LRlc (Lower Risk, least concern. smooth-fronted caiman, Cachirre, Jacaré-coroa Widespread and remains locally abundant, although quantitative data are lacking; IUCN 2009) (last assessed in 1996). Range: Bolivia, Brazil, Colombia, Ecuador, French Guiana, Guyana, Peru, Suriname, Venezuela Principal threats: Habitat destruction, local subsistence hunting, pollution, urbanization Ecology and Natural History The Smooth-fronted caiman is somewhat larger than the dwarf caiman (P. palpebrosus), with a maximum male length of around 2.3 m (Medem 1981). It has a similar distribution to the latter, but does not enter the Brazilian shield region or the Paraguay River drainage. In Brazil, P. trigonatus is found principally in the rivers and streams of heavily forested habitats (Magnusson 1992), in igapó forest in the Central Amazon (Mazurek-Souza 2001), and open water or near waterfalls in the large Rivers such as Mamoré-Madeira- Abunã (Vasconcelos and Campos 2007) and Beni River (Zilca Campos, pers. comm.). In Venezuela, P. trigonatus is principally restricted to chemically poor rivers and streams of the Guyana Shield and western llanos (Godshalk 1982; Gorzula and Paolillo 1986; Gorzula et al. 1988), and has been reported at elevations up to 1300 m. The habitat in Bolivia is similar to that reported for P. palpebrosus (King and Videz- Roca 1989). -

Distribution and Abundance of Four Caiman Species (Crocodylia: Alligatoridae) in Jaú National Park, Amazonas, Brazil*
Rev. Biol. Trop. 49(3-4): 1095-1109, 2001 www.ucr.ac.cr www.ots.ac.cr www.ots.duke.edu Distribution and abundance of four caiman species (Crocodylia: Alligatoridae) in Jaú National Park, Amazonas, Brazil* George Henrique Rebêlo1 and Luciana Lugli2 1 Instituto Nacional de Pesquisas da Amazônia, Coordenação de Pesquisas em Ecologia, Caixa Postal 478, 69011-970 Manaus-AM, Brazil. Phone: 55 92 6431820. E-mail: [email protected] 2 Universidade Estadual Paulista, Rio Claro-SP, Brazil. Phone: 55 19 5332952. E-mail: [email protected] * This study is dedicated to the memory of our friend, the Brazilian herpetologist and political activist Glória Moreira [1963-1997]. Received 17-I-2000. Corrected 03-X-2000. Accepted 08-II-2001. Abstract: Jaú National Park is a large rain forest reserve that contains small populations of four caiman species. We sampled crocodilian populations during 30 surveys over a period of four years in five study areas. We found the mean abundance of caiman species to be very low (1.0 ± 0.5 caiman/km of shoreline), independent of habi- tat type (river, stream or lake) and season. While abundance was almost equal, the species’ composition varied in different waterbody and study areas. We analysed the structure similarity of this assemblage. Lake and river habitats were the most similar habitats, and inhabited by at least two species, mainly Caiman crocodilus and Melanosuchus niger. However, those species can also inhabit streams. Streams were the most dissimilar habi- tats studied and also had two other species: Paleosuchus trigonatus and P. palpebrosus. -

Snakes As Prey of Cuvier's Dwarf Caiman (Paleosuchus
Herpetology Notes, volume 10: 169-170 (2017) (published online on 25 April 2017) Snakes as prey of Cuvier’s Dwarf Caiman (Paleosuchus palpebrosus: Alligatoridae), with a new observation from central Amazonia, Brazil Diogo Dutra-Araújo1,2,*, Boris Marioni2, Rafael de Fraga3 and Ronis da Silveira4 The Dwarf Caiman, Paleosuchus palpebrosus and Campos, 2010). Based on the known diet for other (Cuvier, 1807) is among the smallest and most secretive Amazonian caiman species (Magnusson et al., 1987; crocodilians in the world, and this is the main reason why Da Silveira and Magnusson, 1999) we would expect information on the species’ natural history and ecology P. palpebrosus to have an opportunistic-generalist are still scarce (Magnusson and Campos, 2010). The diet. However, basic information on its diet, such as species is widely distributed in South America, occurring the diversity of prey that could be consumed, foraging in Bolivia, Brazil, Colombia, Ecuador, French Guiana, mode, and the feeding behavior itself are poorly known Guyana, Paraguay, Peru, Suriname, and Venezuela. In (Magnusson, 1985; Magnusson et al., 1987; Campos et Brazil the species occupies widely divergent ecosystems al., 1995; Botero-Arias, 2007). In this study we report (Magnusson, 1992), including Amazonian rainforest, an observation of P. palpebrosus preying on a snake in caatinga (in northeastern Brazil), cerrado (on the central Amazonia. Additionally, we present a revised central plateau), Atlantic Forest, and Pantanal (a large account of the prey types consumed by the species. wetland in western Brazil). Paleosuchus palpebrosus Ontogenetic variation in diet has been reported widely can occupy a wide variety of microhabitats, such as in crocodilians, including P. -

B-73 Crocodiles Et Alligators Captures Par Espèces, Zones De Pêche Et Pays Ou Zones Cocodrilos Y Aligatores Capturas Por Especies, Áreas De Pesca Y Países O Áreas
377 Crocodiles and alligators Capture production by species, fishing areas and countries or areas B-73 Crocodiles et alligators Captures par espèces, zones de pêche et pays ou zones Cocodrilos y aligatores Capturas por especies, áreas de pesca y países o áreas Species, Fishing area Espèce, Zone de pêche 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 Especie, Área de pesca no no no no no no no no no no Broad-nosed caiman ...B ...C Caiman latirostris 5,36(01)001,01 IMO 03 Argentina - - - - - 90 165 215 2 752 880 Brazil - - - - - - - 10 ... 50 03 Fishing area total - - - - - 90 165 225 2 752 930 Species total - - - - - 90 165 225 2 752 930 Spectacled caiman Caïman à lunettes Caimán de anteojos Caiman crocodilus 5,36(01)001,03 CAI 02 Costa Rica - 40 - - - - - - - - Cuba 506 5 - - - - - - - - Nicaragua 1 590 3 927 250 6 440 ... ... ... ... ... ... Panama 500 3 022 10 10 250 11 700 13 298 14 694 15 850 4 696 2 210 02 Fishing area total 2 596 6 994 260 16 690 11 700 13 298 14 694 15 850 4 696 2 210 03 Argentina - - - - - - - 1 1 291 2 203 Bolivia 15 961 1 757 17 500 ... 28 170 31 018 43 528 36 299 51 330 18 296 Brazil 7 307 2 092 4 619 8 286 1 253 6 048 12 851 7 004 620 673 Colombia 452 707 670 389 771 456 832 203 704 313 540 579 555 719 612 041 601 958 975 721 Guyana 910 ... 9 880 9 880 5 917 8 222 3 124 3 310 2 301 3 720 Paraguay 503 4 445 - 9 750 3 793 8 373 4 409 - - - Venezuela 33 528 35 579 24 640 23 655 19 215 20 349 33 942 63 902 58 346 60 864 03 Fishing area total 510 916 714 262 828 095 883 774 762 661 614 589 653 573 722 557 715 -
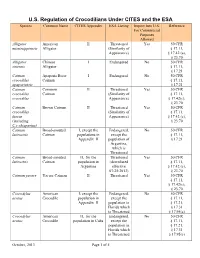
U.S. Regulation of Crocodilians Under CITES and the ESA Species Common Name CITES Appendix ESA Listing Import Into U.S
U.S. Regulation of Crocodilians Under CITES and the ESA Species Common Name CITES Appendix ESA Listing Import Into U.S. Reference For Commercial Purposes Allowed Alligator American II Threatened Yes 50 CFR mississippiensis Alligator (Similarity of § 17.11, Appearance) § 17.42 (a), § 23.70 Alligator Chinese I Endangered No 50 CFR sinensis Alligator § 17.11, § 17.21 Caiman Apaporis River I Endangered No 50 CFR crocodilus Caiman § 17.11, apaporiensis § 17.21 Caiman Common II Threatened Yes 50 CFR crocodilus Caiman (Similarity of § 17.11, crocodilus Appearance) § 17.42(c), § 23.70 Caiman Brown Caiman II Threatened Yes 50 CFR crocodilus (Similarity of § 17.11, fuscus Appearance) § 17.42 (c), (including § 23.70 C.c.chiapasius) Caiman Broad-snouted I, except the Endangered, No 50 CFR latirostris Caiman populations in except the § 17.11, Appendix II population of § 17.21 Argentina, which is Threatened Caiman Broad-snouted II, for the Threatened Yes 50 CFR latirostris Caiman population in (downlisted § 17.11, Argentina effective § 17.42 (c), 07/25/2013) § 23.70 Caiman yacare Yacare Caiman II Threatened Yes 50 CFR § 17.11, § 17.42(c), § 23.70 Crocodylus American I, except the Endangered, No 50 CFR acutus Crocodile population in except the § 17.11, Appendix II population in § 17.21, Florida which § 17.31 is Threatened § 17.95(c) Crocodylus American II, for the Endangered, No 50 CFR acutus Crocodile population in Cuba except the § 17.11, population in § 17.21, Florida which § 17.31 is Threatened § 17.95(c) October, 2013 Page 1 of 5 U.S. Regulation of Crocodilians Under CITES and the ESA Species Common Name CITES Appendix ESA Listing Import Into U.S.