Covered = = from Public Databases (Fig
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abies Alba Mill.) Differ Largely in Mature Silver Fir Stands and in Scots Pine Forecrops Rafal Ważny
Ectomycorrhizal communities associated with silver fir seedlings (Abies alba Mill.) differ largely in mature silver fir stands and in Scots pine forecrops Rafal Ważny To cite this version: Rafal Ważny. Ectomycorrhizal communities associated with silver fir seedlings (Abies alba Mill.) differ largely in mature silver fir stands and in Scots pine forecrops. Annals of Forest Science, Springer Nature (since 2011)/EDP Science (until 2010), 2014, 71 (7), pp.801 - 810. 10.1007/s13595-014-0378-0. hal-01102886 HAL Id: hal-01102886 https://hal.archives-ouvertes.fr/hal-01102886 Submitted on 13 Jan 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Annals of Forest Science (2014) 71:801–810 DOI 10.1007/s13595-014-0378-0 ORIGINAL PAPER Ectomycorrhizal communities associated with silver fir seedlings (Abies alba Mill.) differ largely in mature silver fir stands and in Scots pine forecrops Rafał Ważny Received: 28 August 2013 /Accepted: 14 April 2014 /Published online: 14 May 2014 # The Author(s) 2014. This article is published with open access at Springerlink.com Abstract colonization of seedling roots was similar in both cases. This & Context The requirement for rebuilding forecrop stands suggests that pine stands afforested on formerly arable land besides replacement of meadow vegetation with forest plants bear enough ECM species to allow survival and growth of and formation of soil humus is the presence of a compatible silver fir seedlings. -

Caryophyllales 2018 Instituto De Biología, UNAM September 17-23
Caryophyllales 2018 Instituto de Biología, UNAM September 17-23 LOCAL ORGANIZERS Hilda Flores-Olvera, Salvador Arias and Helga Ochoterena, IBUNAM ORGANIZING COMMITTEE Walter G. Berendsohn and Sabine von Mering, BGBM, Berlin, Germany Patricia Hernández-Ledesma, INECOL-Unidad Pátzcuaro, México Gilberto Ocampo, Universidad Autónoma de Aguascalientes, México Ivonne Sánchez del Pino, CICY, Centro de Investigación Científica de Yucatán, Mérida, Yucatán, México SCIENTIFIC COMMITTEE Thomas Borsch, BGBM, Germany Fernando O. Zuloaga, Instituto de Botánica Darwinion, Argentina Victor Sánchez Cordero, IBUNAM, México Cornelia Klak, Bolus Herbarium, Department of Biological Sciences, University of Cape Town, South Africa Hossein Akhani, Department of Plant Sciences, School of Biology, College of Science, University of Tehran, Iran Alexander P. Sukhorukov, Moscow State University, Russia Michael J. Moore, Oberlin College, USA Compilation: Helga Ochoterena / Graphic Design: Julio C. Montero, Diana Martínez GENERAL PROGRAM . 4 MONDAY Monday’s Program . 7 Monday’s Abstracts . 9 TUESDAY Tuesday ‘s Program . 16 Tuesday’s Abstracts . 19 WEDNESDAY Wednesday’s Program . 32 Wednesday’s Abstracs . 35 POSTERS Posters’ Abstracts . 47 WORKSHOPS Workshop 1 . 61 Workshop 2 . 62 PARTICIPANTS . 63 GENERAL INFORMATION . 66 4 Caryophyllales 2018 Caryophyllales General program Monday 17 Tuesday 18 Wednesday 19 Thursday 20 Friday 21 Saturday 22 Sunday 23 Workshop 1 Workshop 2 9:00-10:00 Key note talks Walter G. Michael J. Moore, Berendsohn, Sabine Ya Yang, Diego F. Registration -

Gymnomyces Xerophilus Sp. Nov. (Sequestrate Russulaceae), an Ectomycorrhizal Associate of Quercus in California
MYCOLOGICAL RESEARCH I I0 (2006) 57 5-582 Gymnomyces xerophilus sp. nov. (sequestrate Russulaceae), an ectomycorrhizal associate of Quercus in California Matthew E. SMITHa1*,James M. TRAPPE~,Dauid M. RIZZOa, Steven I. MILLERC 'Department of Plant Pathology, University of California at Davis, Davis CA 95616, USA b~epartmentof Forest Science, Oregon State University, Camallis, OR 97331-5752, USA CDeparhnentof Botany, University of Wyoming, Laramie, WY 82071, USA ARTICLE INFO ABSTRACT Article history: Gymnomyces xerophilus sp. nov., a sequestrate species in the Russulaceae, is characterized Received 30 August 2005 and descn'bed morphologically as a new species from Quercus-dominated woodlands in Accepted 14 February 2006 California. ITS sequences recovered from healthy, ectomycorrhizal roots of Quercus dougla- Corresponding Editor: Michael Weiss sii and Q. wislizeni matched those of G. xerophfius basidiomata, confirming the ectomycor- -- rhizal status of this fungus. Phylogenetic analysis of the ITS region places G. xerophilus in Keywords: a clade with both agaricoid (Russula in the section Polychromae) and sequestrate (Gymno- Basidiomycota myces, Cystangium) relatives. We include a dichotomous key to the species of Gymnomyces Hypogeous fungi associated with Quercus. ITS O 2006 The British Mycological Society. Published by Elsevier Ltd. All rights reserved. Molecular phylogeny Russula Introduction mycelium, potentially reducing drought stress (Duddridge et al. 1980; Parke et al. 1983). Quercus-dominated ecosystems cover about one third of dali- Although the EM fungi associated with Quercus in California fornia's 404,000bm2(Pavlik et al. 1991). Quercus spp. are well have not been studied extensively, Thiers (1984) and Trappe adapted to the state's extensive areas of dry, Mediterranean and Claridge (2005) suggest that seasonally dry climates exert climate, with at least 7 species considered endemic (Nixon a selection pressure towards a sequestrate fruiting habit in 2002). -
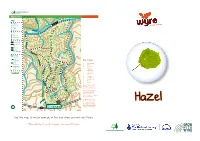
Use This Map to Find an Example of This Tree When You Next Visit Wyre
Wyre Forest Callow Hill area Wimperhill Key Wood Parking 110 rook National Cycle Network s B 45 Information le y w a o ilw D ra d Toilets se su di All access 70 Cafe Play area 90 Picnic area 90 Horse riding New Parks Buzzard Trail Woodpecker 110 Trail 110 7 Black Wren Trail Gate 8 Family 6 Mountain Bike New Parks Park Brook Bench Trail Corner 9 National 120 Cycle Route 6 Public footpaths Key to trees Arboretum 5 4 Public 1 European Larch 8 7 bridleways 3 2 Holly Bore 10 Park 1 Emergency Hole 3 Pool 3 Douglas Fir 9 numbered 3 4 Oak Callow 13 5 Silver Birch posts 13 Hill 6 Wild Service 140 3 0 100 200m 2 7 True Service (Whitty Pear) 16 3 3 8 Ash 9 Scots Pine 2 11 10 Corsican Pine 11 Alder Buckthorn 9 10 5 Doghanging 5 12 Hazel Coppice Woodland 13 Hawthorn Giants 11 NB: Some numbers relate to 15 4 individual trees and some to 13 140 plantations of a single species. 5 1 10 12 10 4 As you learn the trees see if 9 you can spot them at other 12 1 Albert’s Oak locations around the route. 8 150 170 14 (Eg silver birch is very common 3 just about everywhere!) Discovery 160 You will also come across Centre 1 2 different tree species on this Bewdley route - look at their leaves, buds and bark and see if you Hazel Wyre Visitor Centre can identify what they are by Tenbury Wells using a book or the internet. -

Mycodiversity Studies in Selected Ecosystems of Greece: 5
Uploaded — May 2011 [Link page — MYCOTAXON 115: 535] Expert reviewers: Giuseppe Venturella, Solomon P. Wasser Mycodiversity studies in selected ecosystems of Greece: 5. Basidiomycetes associated with woods dominated by Castanea sativa (Nafpactia Mts., central Greece) ELIAS POLEMIS1, DIMITRIS M. DIMOU1,3, LEONIDAS POUNTZAS4, DIMITRIS TZANOUDAKIS2 & GEORGIOS I. ZERVAKIS1* 1 [email protected], [email protected] Agricultural University of Athens, Lab. of General & Agricultural Microbiology Iera Odos 75, 11855 Athens, Greece 2 University of Patras, Dept. of Biology, Panepistimioupoli, 26500 Rion, Greece 3 Koritsas 10, 15343 Agia Paraskevi, Greece 4 Technological Educational Institute of Mesologgi, 30200 Mesologgi, Greece Abstract — Very scarce literature data are available on the macrofungi associated with sweet chestnut trees (Castanea sativa, Fagaceae). We report here the results of an inventory of basidiomycetes, which was undertaken in the region of Nafpactia Mts., central Greece. The investigated area, with woods dominated by C. sativa, was examined for the first time in respect to its mycodiversity. One hundred and four species belonging in 54 genera were recorded. Fifteen species (Conocybe pseudocrispa, Entoloma nitens, Lactarius glaucescens, Lichenomphalia velutina, Parasola schroeteri, Pholiotina coprophila, Russula alutacea, R. azurea, R. pseudoaeruginea, R. pungens, R. vitellina, Sarcodon glaucopus, Tomentella badia, T. fibrosa and Tubulicrinis sororius) are reported for the first time from Greece. In addition, 33 species constitute new habitats/hosts/substrates records. Key words — biodiversity, macromycete, Mediterranean, mushroom Introduction Castanea sativa Mill., Fagaceae (sweet chestnut) generally prefers north- facing slopes where the rainfall is greater than 600 mm, on moderately acid soils (pH 4.5–6.5) with a light texture. It covers ca. -

Influence of Tree Species on Richness and Diversity of Epigeous Fungal
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Archive Ouverte en Sciences de l'Information et de la Communication fungal ecology 4 (2011) 22e31 available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/funeco Influence of tree species on richness and diversity of epigeous fungal communities in a French temperate forest stand Marc BUE´Ea,*, Jean-Paul MAURICEb, Bernd ZELLERc, Sitraka ANDRIANARISOAc, Jacques RANGERc,Re´gis COURTECUISSEd, Benoıˆt MARC¸AISa, Franc¸ois LE TACONa aINRA Nancy, UMR INRA/UHP 1136 Interactions Arbres/Microorganismes, 54280 Champenoux, France bGroupe Mycologique Vosgien, 18 bis, place des Cordeliers, 88300 Neufchaˆteau, France cINRA Nancy, UR 1138 Bioge´ochimie des Ecosyste`mes Forestiers, 54280 Champenoux, France dUniversite´ de Lille, Faculte´ de Pharmacie, F59006 Lille, France article info abstract Article history: Epigeous saprotrophic and ectomycorrhizal (ECM) fungal sporocarps were assessed during Received 30 September 2009 7 yr in a French temperate experimental forest site with six 30-year-old mono-specific Revision received 10 May 2010 plantations (four coniferous and two hardwood plantations) and one 150-year-old native Accepted 21 July 2010 mixed deciduous forest. A total of 331 fungal species were identified. Half of the fungal Available online 6 October 2010 species were ECM, but this proportion varied slightly by forest composition. The replace- Corresponding editor: Anne Pringle ment of the native forest by mono-specific plantations, including native species such as beech and oak, considerably altered the diversity of epigeous ECM and saprotrophic fungi. Keywords: Among the six mono-specific stands, fungal diversity was the highest in Nordmann fir and Conifer plantation Norway spruce plantations and the lowest in Corsican pine and Douglas fir plantations. -

Structural Characterization and Biological Activity of Lactarius Scrobiculatus
Structural characterization and biological activity of Lactarius scrobiculatus Ivana Tomic Thesis for the Master´ degree in Pharmacy 45 study points Department of Pharmaceutical Chemistry School of Pharmacy Faculty of Mathematics and Natural Sciences UNIVERSITY OF OSLO November/2018 II Structural characterization and biological activity of Lactarius scrobiculatus Thesis for Master´ degree in Pharmacy Department for Pharmaceutical chemistry School of Pharmacy Faculty of Mathematics and Natural Sciences University in Oslo Ivana Tomic November 2018 Supervisor: Anne Berit Samuelsen III © Author 2018 Structural characterization and biological activity of Lactarius scrobiculatus Ivana Tomic http://www.duo.uio.no/ Print: Reprosentralen, Universitetet i Oslo IV Acknowledgments The present thesis was carried out at the Departement of Pharmaceutical Chemistry, University of Oslo (UiO), for the Master´s degree in Pharmacy at the University of Oslo. The other institute include Norwegian Centre of Molecular Medicine, where I have performed activity assay. First and foremost, I would like to thank to my supervisor Anne Berit Samuelsen for hers support and guidance throughout my work and useful comments during the writing. Further, I also want to thank Hoai Thi Nguyen and Cristian Winther Wold for help with carrying out GC and GC-MS analysis. Also, I am very thankful to Karl Malterud for help with NMR analysis. Special thanks to Suthajini Yogarajah for her patience and lab support. I would also like to thank to Kari Inngjerdingen for good and helpful Forskningforberedende kurs. My gratitude goes also to Prebens Morth group at NMCC, special to Julia Weikum and Bojana Sredic, who were always kind and helpful. Finally, I would like to express my fabulous thanks to my wonderful parents, my husband and my four sons for their great patience, sacrifice, moral support and encouragement during my master thesis. -

Russulas of Southern Vancouver Island Coastal Forests
Russulas of Southern Vancouver Island Coastal Forests Volume 1 by Christine Roberts B.Sc. University of Lancaster, 1991 M.S. Oregon State University, 1994 A Dissertation Submitted in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY in the Department of Biology © Christine Roberts 2007 University of Victoria All rights reserved. This dissertation may not be reproduced in whole or in part, by photocopying or other means, without the permission of the author. Library and Bibliotheque et 1*1 Archives Canada Archives Canada Published Heritage Direction du Branch Patrimoine de I'edition 395 Wellington Street 395, rue Wellington Ottawa ON K1A0N4 Ottawa ON K1A0N4 Canada Canada Your file Votre reference ISBN: 978-0-494-47323-8 Our file Notre reference ISBN: 978-0-494-47323-8 NOTICE: AVIS: The author has granted a non L'auteur a accorde une licence non exclusive exclusive license allowing Library permettant a la Bibliotheque et Archives and Archives Canada to reproduce, Canada de reproduire, publier, archiver, publish, archive, preserve, conserve, sauvegarder, conserver, transmettre au public communicate to the public by par telecommunication ou par Plntemet, prefer, telecommunication or on the Internet, distribuer et vendre des theses partout dans loan, distribute and sell theses le monde, a des fins commerciales ou autres, worldwide, for commercial or non sur support microforme, papier, electronique commercial purposes, in microform, et/ou autres formats. paper, electronic and/or any other formats. The author retains copyright L'auteur conserve la propriete du droit d'auteur ownership and moral rights in et des droits moraux qui protege cette these. -

Key to Alberta Edible Mushrooms Note: Key Should Be Used With"Mushrooms of Western Canada"
Key to Alberta Edible Mushrooms Note: Key should be used with"Mushrooms of Western Canada". The key is designed to help narrow the field of possibilities. Should never be used without more detailed descriptions provided in field guides. Always confirm your choice with a good field guide. Go A Has pores or sponge like tubes on underside 2 to 1 Go B Does not have visible pores or sponge like tubes 22 to Leatiporus sulphureous A Bright yellow top, brighter pore surface, shelf like growth on wood "Chicken of the woods" 2 Go B not as above with pores or sponge like tubes 3 to Go A Has sponge like tube layer easily separated from cap 4 to 3 B Has shallow pore layer not easily separated from cap Not described in this key A Medium to large brown cap, thick stalk, fine embossed netting on stalk Boletus edulis 4 Go B Not as above with sponge like tube layer 5 to A Dull brown to beige cap, fine embossed netting on stalk Not described in this key 5 Go B Not as above with sponge like tube layer 6 to Go A Dry cap, rough ornamented stem, with flesh staining various shades of pink to gray 7 to 6 Go B Not as above 12 to Go A Cap orange to red, never brown or white 8 to 7 Go B Cap various shades of dark or light brown to beige/white 10 to A Dark orangey red cap, velvety cap surface, growing exclusively with conifers Leccinum fibrilosum 8 Go B Orangey cap, growing in mixed or pure aspen poplar stands 9 to Orangey - red cap, skin flaps on cap margins, slowly staining pinkish gray, earliest of the leccinums starting A Leccinum boreale in June. -

IL MONDO DEI FUNGHI Appunti Di Micologia
Maria Rosaria Tieri – Nino Tieri IL MONDO DEI FUNGHI appunti di micologia 1 Collana : “I quaderni della natura ” © Dispensa tratta da : FUNGHI D‟ABRUZZO Edizioni Paper's World S. Atto Teramo di Maria Rosaria Tieri e Nino Tieri FUNGHI IN CUCINA Edizioni Menabò di Maria Rosaria Tieri e Nino Tieri Con la preziosa collaborazione del prof. Mimmo Bernabeo Copertina di Nino Tieri © I diritti sono riservati. Il divieto di riproduzione è totale, anche a mezzo fotocopia e per uso interno. Nessuna parte di questa pubblicazione potrà essere riprodotta, archiviata in sistemi di ricerca o trasmessa in qualunque forma elettronica, meccanica, registrata o altro. 2 BREVE STORIA DELLA MICOLOGIA Le origini dei funghi sono di sicuro antichissime, di certo, i funghi, come organismi eucarioti, apparvero sulla terra più di 500 milioni di anni fa. La documentazione, circa la loro presenza, viene dedotta dai resti fossili venuti recente- mente alla luce, risalenti a moltissimi milioni di anni fa: nei resti del carbonifero (300 milioni di anni fa) sono, infatti, riconoscibili varietà di funghi ancora oggi presenti tra le specie della flora fungina. Le popolazioni primordiali, agli inizi della civiltà umana, hanno avuto sicuramente di- mestichezza con i funghi, sia per scopi alimentari che per pratiche religiose ed arti- stiche. Oggi non siamo a conoscenza del significato che i funghi rappresentavano per l‟uomo primitivo. Non è noto, infatti, se egli se ne nutrisse o se li ignorasse, né tanto- meno se fosse in grado di distinguere le specie eduli da quelle velenose. Tra gli oggetti ritrovati nello zaino dell‟uomo di Similaun, risalente a più di 5000 anni fa, vi erano anche funghi allucinogeni secchi. -

One Step Closer to Unravelling the Origin of Russula: Subgenus Glutinosae Subg
Mycosphere 11(1): 285–304 (2020) www.mycosphere.org ISSN 2077 7019 Article Doi 10.5943/mycosphere/11/1/6 One step closer to unravelling the origin of Russula: subgenus Glutinosae subg. nov. Buyck B1*, Wang X-H2, Adamčíková K3, Caboň M4, Jančovičová S5, 6 4 Hofstetter V and Adamčík S 1Institut pour la Systématique, Evolution, Biodiversité (ISYEB), UMR 7205, Case Postale 39 Muséum national d’histoire naturelle, Sorbonne Université, CNRS, 12 Rue Buffon, F-75005 Paris, France 2CAS Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, P. R. China 3Department of Plant Pathology and Mycology, Institute of Forest Ecology, Slovak Academy of Sciences Zvolen, Akademická 2, SK-949 01 Nitra, Slovakia 4Institute of Botany, Plant Science and Biodiversity Center, Slovak Academy of Sciences, Dúbravská cesta 9, SK-845 23 Bratislava, Slovakia 5Department of Botany, Faculty of Natural Sciences, Comenius University in Bratislava, Révová 39, SK-811 02 Bratislava, Slovakia 6Agroscope Research Station, Department of plant protection, Rte de Duiller 60, 1260 Nyon 1, Switzerland Buyck B, Wang X-H, Adamčíková K, Caboň M, Jančovičová S, Hofstetter V, Adamčík S 2020 – One step closer to unravelling the origin of Russula: subgenus Glutinosae subg. nov. Mycosphere 11(1), 285–304, Doi 10.5943/mycosphere/11/1/6 Abstract This study reports on the discovery of a new subgenus, Russula subg. Glutinosae, having an Eastern North American – East Asian distribution. A multigene phylogeny places this new subgenus sister with strong support to a well-supported clade composed of subgenera Compactae and Archaeae. -

Angiocarpous Representatives of the Russulaceae in Tropical South East Asia
Persoonia 32, 2014: 13–24 www.ingentaconnect.com/content/nhn/pimj RESEARCH ARTICLE http://dx.doi.org/10.3767/003158514X679119 Tales of the unexpected: angiocarpous representatives of the Russulaceae in tropical South East Asia A. Verbeken1, D. Stubbe1,2, K. van de Putte1, U. Eberhardt³, J. Nuytinck1,4 Key words Abstract Six new sequestrate Lactarius species are described from tropical forests in South East Asia. Extensive macro- and microscopical descriptions and illustrations of the main anatomical features are provided. Similarities Arcangeliella with other sequestrate Russulales and their phylogenetic relationships are discussed. The placement of the species gasteroid fungi within Lactarius and its subgenera is confirmed by a molecular phylogeny based on ITS, LSU and rpb2 markers. hypogeous fungi A species key of the new taxa, including five other known angiocarpous species from South East Asia reported to Lactarius exude milk, is given. The diversity of angiocarpous fungi in tropical areas is considered underestimated and driving Martellia evolutionary forces towards gasteromycetization are probably more diverse than generally assumed. The discovery morphology of a large diversity of angiocarpous milkcaps on a rather local tropical scale was unexpected, and especially the phylogeny fact that in Sri Lanka more angiocarpous than agaricoid Lactarius species are known now. Zelleromyces Article info Received: 2 February 2013; Accepted: 18 June 2013; Published: 20 January 2014. INTRODUCTION sulales species (Gymnomyces lactifer B.C. Zhang & Y.N. Yu and Martellia ramispina B.C. Zhang & Y.N. Yu) and Tao et al. Sequestrate and angiocarpous basidiomata have developed in (1993) described Martellia nanjingensis B. Liu & K. Tao and several groups of Agaricomycetes.