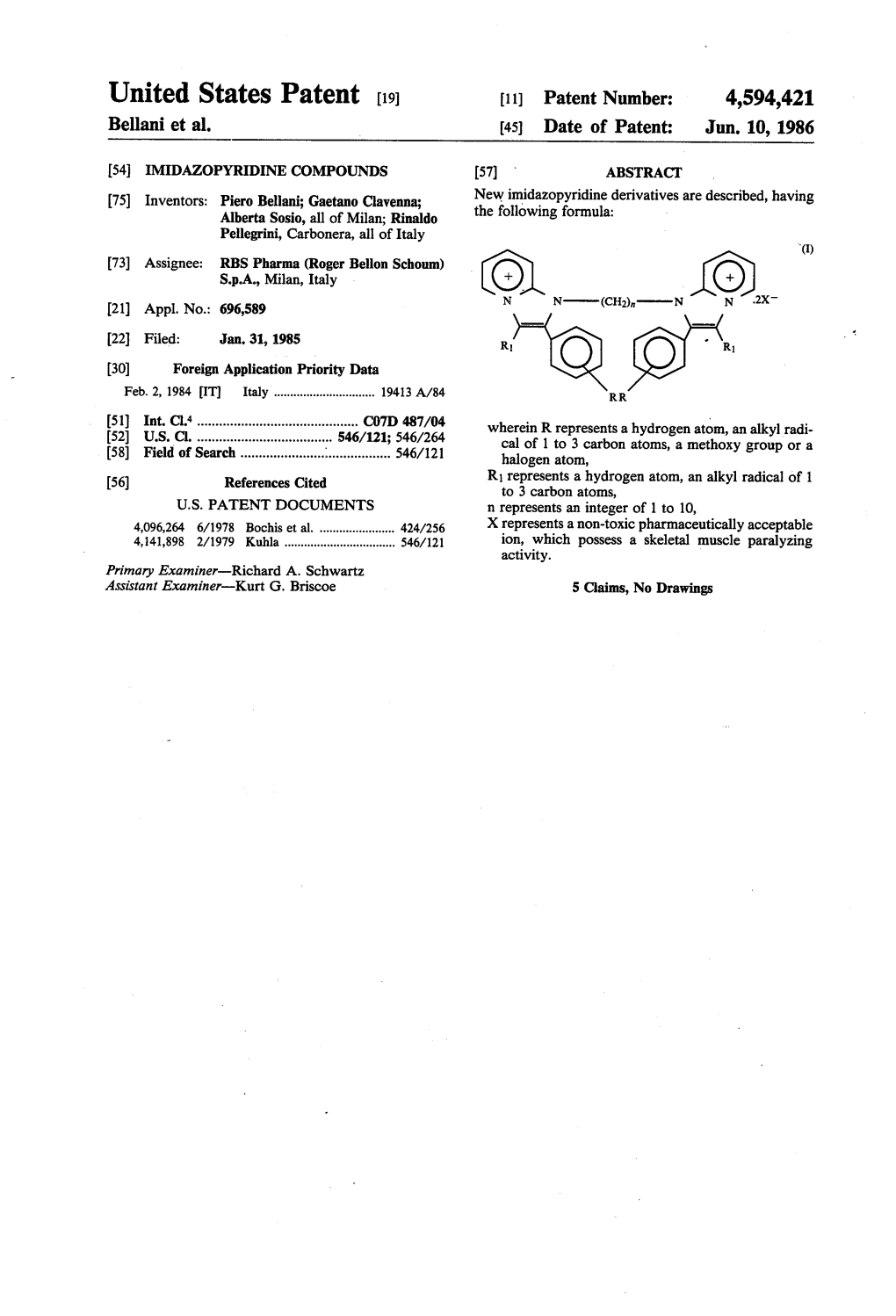
United States Patent [191 4,594,421
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Thesis Submitted for the Degree of Doctor of Philosophy in The
A thesis submitted for the Degree of Doctor of Philosophy in the University of London METABOLIC AND DISPOSITION STUDIES Oy QUATERNARY AMMONIUM COMPOUNDS IN MAN AND LABORATORY SPECIES by JOSEPH D'SOUZA, B.Sc. Department of Biochemical and Experimental Pharmacology, St Mary's Hospital Medical School, London W2 1PG December 1979 -2- To My Parents with Love & Gratitude -3- ABSTRACT The work described in this thesis deals with two aspects of the biochemical pharmacology of quaternary ammonium compounds. The first part deals with the disposition of a quaternary ammonium neuromuscular blocking drug, fazadinium bromide. A convenient radiometric assay for this drug in biological fluids was developed, and applied to the study of its disposition in patients with normal and impaired renal function. The second part describes a study of the metabolic N- methylation and hence quaternisation, of 14C -pyridine in vivo. The main findings are as follows: - A) Disposition studies in the dog and man showed that biliary excretion is an important pathway for the elimination of fazadinium. i) The ratio of the hepatic to renal excretion being 3:1 in the dog, whilst in man, faecal elimination arising presumably from biliary excretion accounted for over one-third the total elimination. ii) Biliary excretion was not augmented to compensate for renal dysfunction after bilateral renal pedicle ligation in the dog; metabolic studies showed the major metabolite in the urine and bile of dogs was 3-methyl-2-phenylimidazole (1,2a) pyridinium, but an "unknown" metabolite in human urine was also detected. B) Clinical studies demonstrated no significant alterations in the pharmacokinetic or pharmacodynamic properties of fazadinium in patients with normal or impaired renal function. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -

S1 Table. List of Medications Analyzed in Present Study Drug
S1 Table. List of medications analyzed in present study Drug class Drugs Propofol, ketamine, etomidate, Barbiturate (1) (thiopental) Benzodiazepines (28) (midazolam, lorazepam, clonazepam, diazepam, chlordiazepoxide, oxazepam, potassium Sedatives clorazepate, bromazepam, clobazam, alprazolam, pinazepam, (32 drugs) nordazepam, fludiazepam, ethyl loflazepate, etizolam, clotiazepam, tofisopam, flurazepam, flunitrazepam, estazolam, triazolam, lormetazepam, temazepam, brotizolam, quazepam, loprazolam, zopiclone, zolpidem) Fentanyl, alfentanil, sufentanil, remifentanil, morphine, Opioid analgesics hydromorphone, nicomorphine, oxycodone, tramadol, (10 drugs) pethidine Acetaminophen, Non-steroidal anti-inflammatory drugs (36) (celecoxib, polmacoxib, etoricoxib, nimesulide, aceclofenac, acemetacin, amfenac, cinnoxicam, dexibuprofen, diclofenac, emorfazone, Non-opioid analgesics etodolac, fenoprofen, flufenamic acid, flurbiprofen, ibuprofen, (44 drugs) ketoprofen, ketorolac, lornoxicam, loxoprofen, mefenamiate, meloxicam, nabumetone, naproxen, oxaprozin, piroxicam, pranoprofen, proglumetacin, sulindac, talniflumate, tenoxicam, tiaprofenic acid, zaltoprofen, morniflumate, pelubiprofen, indomethacin), Anticonvulsants (7) (gabapentin, pregabalin, lamotrigine, levetiracetam, carbamazepine, valproic acid, lacosamide) Vecuronium, rocuronium bromide, cisatracurium, atracurium, Neuromuscular hexafluronium, pipecuronium bromide, doxacurium chloride, blocking agents fazadinium bromide, mivacurium chloride, (12 drugs) pancuronium, gallamine, succinylcholine -

Pharmacological and Clinical Study of the New Non-Depolarizing Muscle Relaxant Vecuroniijm (Org Nc 45 Norcuron)
PHARMACOLOGICAL _AND CLINICAL STUDY OF THE NEW NON-DEPOLARIZING MUSCLE RELAXANT VECURONIIJM (ORG NC 45 NORCURON) .. THeSIS Submitted in Partial Fulfilment for the Degree of M.D. in AnaesthesiM \\\\~\\,\\\\\\ GN:8 BY 616.E p .>'_""..-:.JI Essam Abdel Az.z.im EL Ghobashi M.B., B.Ch., M. Sc , (Anae5thesia) ..... ~uperV J 50~5 Prof. Dr. Prof. Dr. :naam fouad Gadallah r of. and Head of Anaesthesia Mastafa Bayomi Hasanein nd Intensive Care Department, Prof. of Anaesthesia and Intensive Benha facult~ of Medicine Care, Benha FacultH of Medicine Zagazig Universitg Zagazig Unjversit~ Prof. Dr. Mahmoud f-Iamdi Mohamed Prof. and Head of Pharmacologg Department, Benha Facult!:j of Medicine Zagazig Universitg 1992 INTRODUCTION AND AIM OF WORK Muscle relaxants made genefalI anaesthesla more easy and effective, facilitated surgery and redued post-operative morbidity and m~rtality. Th~ir univers al rola in control l e d ventilation has contributed to the remarkable improvement in the manageme nt and prognosis of diseases associate d with respiratory failure, e.g. convulsions { tetanus, status epilepticus }, drug over dosage, head, chest injuries, and in the intensive care units. The ideal muscle relaxants should have the following properties:- (K~ and GL~, 1971) 1 It should be competitive and non-depolarizing, thus side effects associated with depolarizers should not occur. 2 The cessation of the neuromuscular blorkade should not depend on renal or hepatic function, so diseases of one or both of them do not impair its elimination. 3 The breakdown products should not posses neuromuscular. blocking effects or other side effects. ~- Relative rapid onset and short duration of action. -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

PHARMACEUTICAL APPENDIX to the HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2008) (Rev
Harmonized Tariff Schedule of the United States (2008) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2008) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM GADOCOLETICUM 280776-87-6 ABAFUNGIN 129639-79-8 ACIDUM LIDADRONICUM 63132-38-7 ABAMECTIN 65195-55-3 ACIDUM SALCAPROZICUM 183990-46-7 ABANOQUIL 90402-40-7 ACIDUM SALCLOBUZICUM 387825-03-8 ABAPERIDONUM 183849-43-6 ACIFRAN 72420-38-3 ABARELIX 183552-38-7 ACIPIMOX 51037-30-0 ABATACEPTUM 332348-12-6 ACITAZANOLAST 114607-46-4 ABCIXIMAB 143653-53-6 ACITEMATE 101197-99-3 ABECARNIL 111841-85-1 ACITRETIN 55079-83-9 ABETIMUSUM 167362-48-3 ACIVICIN 42228-92-2 ABIRATERONE 154229-19-3 ACLANTATE 39633-62-0 ABITESARTAN 137882-98-5 ACLARUBICIN 57576-44-0 ABLUKAST 96566-25-5 ACLATONIUM NAPADISILATE 55077-30-0 ABRINEURINUM 178535-93-8 ACODAZOLE 79152-85-5 ABUNIDAZOLE 91017-58-2 ACOLBIFENUM 182167-02-8 ACADESINE 2627-69-2 ACONIAZIDE 13410-86-1 ACAMPROSATE -

POISONS LIST APPENDIX Amoebicides: Carbarsone
POISONS LIST APPENDIX Amoebicides: Carbarsone Clioquinol and other halogenated hydroxyquinoline compounds Dehydroemetine; its salts Diloxanide; its compounds Dimetridazole Emetine Ipronidazole Metronidazole Pentamidine; its salts Ronidazole Anaesthetics: Alphadolone acetate Alphaxolone Desflurane Disoprofol Enflurane Ethyl ether Etomidate; its salts Halothane Isoflurane Ketamine; its salts Local anaesthetics, the following: their salts; their homologues and analogues; their molecular compounds Amino-alcohols esterified with benzoic acid, phenylacetic acid, phenylpropionic acid, cinnamic acid or the derivatives of these acids; their salts Benzocaine Bupivacaine Butyl aminobenzoate Cinchocaine Diperodon Etidocaine Levobupivacaine Lignocaine Mepivacaine Orthocaine Oxethazaine Phenacaine Phenodianisyl Prilocaine Ropivacaine Phencyclidine; its salts Propanidid Sevoflurane Tiletamine; its salts Tribromoethanol Analeptics and Central Stimulants: Amiphenazole; its salts Amphetamine (DD) Bemegride Cathine Cathinone (DD) Dimethoxybromoamphetamine (DOB) (DD) 2, 5-Dimethoxyamphetamine (DMA) (DD) 2, 5-Dimethoxy-4-ethylamphetamine (DOET) (DD) Ethamivan N-Ethylamphetamine; its salts N-Ethyl MDA (DD) N-Hydroxy MDA (DD) Etryptamine (DD) Fencamfamine Fenetylline Lefetamine or SPA or (-)-1-dimethylamino-1, 2-diphenylethane Leptazol Lobelia, alkaloids of Meclofenoxate; its salts Methamphetamine (DD) Methcathinone (DD) 5-Methoxy-3, 4-methylenedioxyamphetamine (MMDA) (DD) Methylenedioxyamphetamine (MDA) (DD) 3, 4-Methylenedioxymetamphetamine (MDMA) (DD) Methylphenidate; -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

O Brometo De Fazadinium (AH 8165) É Um Bloqueador· Neuromuscular Não
Revista Brasileira de Anestesiologia - Ano 28 - N.0 3 - Maio-Jun., 197s; AÇÃO DO BROMETO DE FAZADINIUM (AH 8165) SOBRE; A JUN·ÇAO MIONEURAL DE CAES NORMAIS E NEFRECTOMIZADOS ( *) r DRA. EUGESSE CREMONESI ( **) DRA. EUZA CREMONESI ( *>-'* ) DRA. ILDA DE JESUS RODRIGUES ( * >!: **), Foi analisado o efeito do brometo de f &i,adinium (AH 8165) , ad1ninistrado por via venosa, na dose de 0,5 mg/ kg, sobre a j1t1ição mioneural de 10 cães n orm ais e 10 cães submetidos à 11,efrectomia bilateral recent e. O agente induziu relaxamento muscular reversível espon ) tâneamente, nos cães normais e nefrectomizados previame1ite. ~ Nos primeiros, a duração da curarização var iou de 13 a 30 m i rr- nutos (m édia de 22,5 min.) e n os ú ltimos var iou de 12 a 55 m i:nutos (1nédia de 25,5 min.). A diferença de m édias não foi significante, quando analisada estatisticame1ite pelo test e T de Student. Os resultados, obtidos em cães, 1ião podem ser transpostos co1n segurança para o homem, devido à diferença bi0cinética da droga. • • O brometo de fazadinium (AH 8165) é um bloqueador· neuromuscular n ão despolarizante (1º) , com início de ação p1·onta e duração curta em cães e gatos e mais prolongada em ·macacos (1 ). - No homem, a duraça-o de seu efeito é semelhante à do b1·ometo de ipancurônio ( u, ) . ( *) T rabalho realizado no Laboro.tório de Anestesiologia Experimental da Dis ciplina de Terapêutica Clín ica da Faculdade de Medicil1a d a Universidade de São Paulo. (**) Professora Livre Docente. (*.,*) Assistente Voluntário. \ ****) Preparador. • ,·ecebido em 9/ 12/ 77 aprovado p/ publi1cação em 26/ 12/ 77 REVISTA BRASILEIRA DE ANESTESIOLOGIA 303 A droga determina, em animais e no homem, um bloqueio ganglionar (em doses elevada) e vagai, induzindo taquicardia intensa (º·º·'). -

Methylnaltrexone Bromide
含溴药物品种资料汇编 药智数据 编纂 简要说明: 本资料汇编由正文 100 页,附件 112 页构成。正文部分汇总了全部含溴药物, 包括曾经研究过、曾经上市应用、目前尚在应用以及目前尚在研的含溴药物,计 259 种。对于其中 15 种重点药物,在附件部分给出其合成方法、研发背景、药理、 药代、安全性、临床试验等方面详细的研究结果。 著录体例: 1,【别 名】,包括药物的试验编号、商标名称及其英文异名; 2,【名称来源】,指的是 WHO DI 网站上公布的推荐 INN 表和建议 INN 表, 例如,pINN-073,1995 rINN-036,1996 表示该 INN 名称来自于 1995 年的推荐 INN 第 73 表和 1996 年的建议 INN 第 36 表。 3,【中文 CADN】,指的是经国家药典委员会确定的中国药品通用名称,名 后注(97),表示该名称见于《中国药品通用名称》(1997 年版);名后注(GB4), 表示该名称见于《国家药品标准工作手册》(第四版)。 4,【建议 CADN】,指的是第 3 条中所述两书均未收录,由《药智数据》根据 INN 和 CADN 命名规律而自拟的药品中文译名。 【英文 INN】acebrochol 【别 名】acebrocol 【名称来源】pINN-001,1953 rINN-001,1955 【中文 CADN】醋溴考尔 (97) 【建议 CADN】 【化学表述】Cholestan-3-ol, 5,6-dibromo-, acetate, (3β,5α,6β)- 【CA 登记号】[514-50-1] 【分 子 式】C29H48Br2O2 【结 构 式】 【品种类别】神经系统>催眠镇静药>其它 【英文 INN】azamethonium bromide 【别 名】 【名称来源】pINN-001,1953 rINN-001,1955 【中文 CADN】阿扎溴铵(GB4) 【建议 CADN】 【化学表述】3-methyl-3-azapentane-1,5-bis(ethyl dimethyl ammonium) bromide 【CA 登记号】[306-53-6] 【分 子 式】C13H33Br2N3 【结 构 式】 【品种类别】心血管系统>抗高血压药>双季铵盐类 【英文 INN】benzpyrinium bromide 【别 名】 【名称来源】pINN-001,1953 rINN-001,1955 【中文 CADN】苄吡溴铵(GB4) 【建议 CADN】 【化学表述】Pyridinium, 1-benzyl-3-(dimethylcarbamyloxy)-, bromide 【CA 登记号】[587-46-2] 【分 子 式】C15H17BrN2O2 【结 构 式】 【品种类别】拟胆碱药 英文 INN】bibrocathol 【别 名】Noviform 【名称来源】pINN-001,1953 rINN-001,1955 【中文 CADN】铋溴酚(97) 【建议 CADN】 【化学表述】4,5,6,7-Tetrabromo-2-hydroxy-1,3,2-Benzodioxabismole 【CA 登记号】[6915-57-7] 【分 子 式】C6HBiBr4O3 【结 构 式】 【品种类别】眼科用药>消毒防腐药>其它 【英文 INN】carbromal 【别 名】 【名称来源】pINN-001,1953 rINN-001,1955 【中文 CADN】卡溴脲(97) 【建议 CADN】 【化学表述】Butanamide, N-(aminocarbonyl)-2-bromo-2-ethyl- 【CA 登记号】[77-65-6] -

A New Non-Depolarizing Neuromuscular Blocking Agent
STUDIES ON FAZADINIUM BROMIDE (AH 8165): A NEW NON-DEPOLARIZING NEUROMUSCULAR BLOCKING AGENT R. HUCHES, J.P. PAYNE, AND N. SUGAI INTRODUCTION PRELIMINARY REPORTS on the neuromuscular blocking action of fazadinium (AH 8165) demonstrated that it is a non-depolarizing agent, that its onset of action is rapid and that its duration is short in cats and dogs but longer in monkeys.1 In man, its duration of action was longer than expected and resembled that in monkeys; persistence of action in man was comparable with that of pancuronium. 2 In cats, Marshall 3 showed that ganglionic blockade was also produced by fazadinium but only with doses greater than those required to cause neuromuscular paralysis. Vagal blockade was caused, however, by doses less than those required for neuromuscular paralysis and it seems likely that such atropinic effects may lead to undesirable tachycardia in man. 4 Indeed, it has already been reported that heart rate and cardiac output increase significantly after fazadinium in man but that arterial blood pressure remains steady.5 The studies reported here were carried out in anaesthetized cats, monkeys and man to investigate further the neuromuscular, cardiovascular and autonomic effects of fazadinium. METHODS Experimental animals. Four cats weighing between 2.8 and 4.8 kg were studied after anaesthesia was induced with 4--8 per cent halothane and maintained with chloralose (60 to 80 mg/kg iv) after cannulation of a iugular vein as described previously.4,8 The trachea was cannulated to allow artificial ventilation and to record the respiratory pattern. The carotid artery was cannulated to measure the blood pressure and the cannula inserted in the jugular vein allowed the administra- tion of drugs.