Knots and Links Arising from Recombination 12379
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Borromean Rings: a Video About the New IMU Logo
The Borromean Rings: A Video about the New IMU Logo Charles Gunn and John M. Sullivan∗ Technische Universitat¨ Berlin Institut fur¨ Mathematik, MA 3–2 Str. des 17. Juni 136 10623 Berlin, Germany Email: {gunn,sullivan}@math.tu-berlin.de Abstract This paper describes our video The Borromean Rings: A new logo for the IMU, which was premiered at the opening ceremony of the last International Congress. The video explains some of the mathematics behind the logo of the In- ternational Mathematical Union, which is based on the tight configuration of the Borromean rings. This configuration has pyritohedral symmetry, so the video includes an exploration of this interesting symmetry group. Figure 1: The IMU logo depicts the tight config- Figure 2: A typical diagram for the Borromean uration of the Borromean rings. Its symmetry is rings uses three round circles, with alternating pyritohedral, as defined in Section 3. crossings. In the upper corners are diagrams for two other three-component Brunnian links. 1 The IMU Logo and the Borromean Rings In 2004, the International Mathematical Union (IMU), which had never had a logo, announced a competition to design one. The winning entry, shown in Figure 1, was designed by one of us (Sullivan) with help from Nancy Wrinkle. It depicts the Borromean rings, not in the usual diagram (Figure 2) but instead in their tight configuration, the shape they have when tied tight in thick rope. This IMU logo was unveiled at the opening ceremony of the International Congress of Mathematicians (ICM 2006) in Madrid. We were invited to produce a short video [10] about some of the mathematics behind the logo; it was shown at the opening and closing ceremonies, and can be viewed at www.isama.org/jms/Videos/imu/. -

Ribbon Concordances and Doubly Slice Knots
Ribbon concordances and doubly slice knots Ruppik, Benjamin Matthias Advisors: Arunima Ray & Peter Teichner The knot concordance group Glossary Definition 1. A knot K ⊂ S3 is called (topologically/smoothly) slice if it arises as the equatorial slice of a (locally flat/smooth) embedding of a 2-sphere in S4. – Abelian invariants: Algebraic invari- Proposition. Under the equivalence relation “K ∼ J iff K#(−J) is slice”, the commutative monoid ants extracted from the Alexander module Z 3 −1 A (K) = H1( \ K; [t, t ]) (i.e. from the n isotopy classes of o connected sum S Z K := 1 3 , # := oriented knots S ,→S of knots homology of the universal abelian cover of the becomes a group C := K/ ∼, the knot concordance group. knot complement). The neutral element is given by the (equivalence class) of the unknot, and the inverse of [K] is [rK], i.e. the – Blanchfield linking form: Sesquilin- mirror image of K with opposite orientation. ear form on the Alexander module ( ) sm top Bl: AZ(K) × AZ(K) → Q Z , the definition Remark. We should be careful and distinguish the smooth C and topologically locally flat C version, Z[Z] because there is a huge difference between those: Ker(Csm → Ctop) is infinitely generated! uses that AZ(K) is a Z[Z]-torsion module. – Levine-Tristram-signature: For ω ∈ S1, Some classical structure results: take the signature of the Hermitian matrix sm top ∼ ∞ ∞ ∞ T – There are surjective homomorphisms C → C → AC, where AC = Z ⊕ Z2 ⊕ Z4 is Levine’s algebraic (1 − ω)V + (1 − ω)V , where V is a Seifert concordance group. -

A Knot-Vice's Guide to Untangling Knot Theory, Undergraduate
A Knot-vice’s Guide to Untangling Knot Theory Rebecca Hardenbrook Department of Mathematics University of Utah Rebecca Hardenbrook A Knot-vice’s Guide to Untangling Knot Theory 1 / 26 What is Not a Knot? Rebecca Hardenbrook A Knot-vice’s Guide to Untangling Knot Theory 2 / 26 What is a Knot? 2 A knot is an embedding of the circle in the Euclidean plane (R ). 3 Also defined as a closed, non-self-intersecting curve in R . 2 Represented by knot projections in R . Rebecca Hardenbrook A Knot-vice’s Guide to Untangling Knot Theory 3 / 26 Why Knots? Late nineteenth century chemists and physicists believed that a substance known as aether existed throughout all of space. Could knots represent the elements? Rebecca Hardenbrook A Knot-vice’s Guide to Untangling Knot Theory 4 / 26 Why Knots? Rebecca Hardenbrook A Knot-vice’s Guide to Untangling Knot Theory 5 / 26 Why Knots? Unfortunately, no. Nevertheless, mathematicians continued to study knots! Rebecca Hardenbrook A Knot-vice’s Guide to Untangling Knot Theory 6 / 26 Current Applications Natural knotting in DNA molecules (1980s). Credit: K. Kimura et al. (1999) Rebecca Hardenbrook A Knot-vice’s Guide to Untangling Knot Theory 7 / 26 Current Applications Chemical synthesis of knotted molecules – Dietrich-Buchecker and Sauvage (1988). Credit: J. Guo et al. (2010) Rebecca Hardenbrook A Knot-vice’s Guide to Untangling Knot Theory 8 / 26 Current Applications Use of lattice models, e.g. the Ising model (1925), and planar projection of knots to find a knot invariant via statistical mechanics. Credit: D. Chicherin, V.P. -

On the Number of Unknot Diagrams Carolina Medina, Jorge Luis Ramírez Alfonsín, Gelasio Salazar
On the number of unknot diagrams Carolina Medina, Jorge Luis Ramírez Alfonsín, Gelasio Salazar To cite this version: Carolina Medina, Jorge Luis Ramírez Alfonsín, Gelasio Salazar. On the number of unknot diagrams. SIAM Journal on Discrete Mathematics, Society for Industrial and Applied Mathematics, 2019, 33 (1), pp.306-326. 10.1137/17M115462X. hal-02049077 HAL Id: hal-02049077 https://hal.archives-ouvertes.fr/hal-02049077 Submitted on 26 Feb 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. On the number of unknot diagrams Carolina Medina1, Jorge L. Ramírez-Alfonsín2,3, and Gelasio Salazar1,3 1Instituto de Física, UASLP. San Luis Potosí, Mexico, 78000. 2Institut Montpelliérain Alexander Grothendieck, Université de Montpellier. Place Eugèene Bataillon, 34095 Montpellier, France. 3Unité Mixte Internationale CNRS-CONACYT-UNAM “Laboratoire Solomon Lefschetz”. Cuernavaca, Mexico. October 17, 2017 Abstract Let D be a knot diagram, and let D denote the set of diagrams that can be obtained from D by crossing exchanges. If D has n crossings, then D consists of 2n diagrams. A folklore argument shows that at least one of these 2n diagrams is unknot, from which it follows that every diagram has finite unknotting number. -
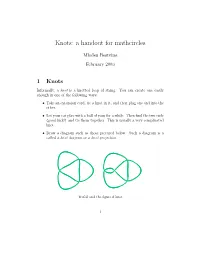
Knots: a Handout for Mathcircles
Knots: a handout for mathcircles Mladen Bestvina February 2003 1 Knots Informally, a knot is a knotted loop of string. You can create one easily enough in one of the following ways: • Take an extension cord, tie a knot in it, and then plug one end into the other. • Let your cat play with a ball of yarn for a while. Then find the two ends (good luck!) and tie them together. This is usually a very complicated knot. • Draw a diagram such as those pictured below. Such a diagram is a called a knot diagram or a knot projection. Trefoil and the figure 8 knot 1 The above two knots are the world's simplest knots. At the end of the handout you can see many more pictures of knots (from Robert Scharein's web site). The same picture contains many links as well. A link consists of several loops of string. Some links are so famous that they have names. For 2 2 3 example, 21 is the Hopf link, 51 is the Whitehead link, and 62 are the Bor- romean rings. They have the feature that individual strings (or components in mathematical parlance) are untangled (or unknotted) but you can't pull the strings apart without cutting. A bit of terminology: A crossing is a place where the knot crosses itself. The first number in knot's \name" is the number of crossings. Can you figure out the meaning of the other number(s)? 2 Reidemeister moves There are many knot diagrams representing the same knot. For example, both diagrams below represent the unknot. -

A Remarkable 20-Crossing Tangle Shalom Eliahou, Jean Fromentin
A remarkable 20-crossing tangle Shalom Eliahou, Jean Fromentin To cite this version: Shalom Eliahou, Jean Fromentin. A remarkable 20-crossing tangle. 2016. hal-01382778v2 HAL Id: hal-01382778 https://hal.archives-ouvertes.fr/hal-01382778v2 Preprint submitted on 16 Jan 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. A REMARKABLE 20-CROSSING TANGLE SHALOM ELIAHOU AND JEAN FROMENTIN Abstract. For any positive integer r, we exhibit a nontrivial knot Kr with r− r (20·2 1 +1) crossings whose Jones polynomial V (Kr) is equal to 1 modulo 2 . Our construction rests on a certain 20-crossing tangle T20 which is undetectable by the Kauffman bracket polynomial pair mod 2. 1. Introduction In [6], M. B. Thistlethwaite gave two 2–component links and one 3–component link which are nontrivial and yet have the same Jones polynomial as the corre- sponding unlink U 2 and U 3, respectively. These were the first known examples of nontrivial links undetectable by the Jones polynomial. Shortly thereafter, it was shown in [2] that, for any integer k ≥ 2, there exist infinitely many nontrivial k–component links whose Jones polynomial is equal to that of the k–component unlink U k. -

Splitting Numbers of Links
Splitting numbers of links Jae Choon Cha, Stefan Friedl, and Mark Powell Department of Mathematics, POSTECH, Pohang 790{784, Republic of Korea, and School of Mathematics, Korea Institute for Advanced Study, Seoul 130{722, Republic of Korea E-mail address: [email protected] Mathematisches Institut, Universit¨atzu K¨oln,50931 K¨oln,Germany E-mail address: [email protected] D´epartement de Math´ematiques,Universit´edu Qu´ebec `aMontr´eal,Montr´eal,QC, Canada E-mail address: [email protected] Abstract. The splitting number of a link is the minimal number of crossing changes between different components required, on any diagram, to convert it to a split link. We introduce new techniques to compute the splitting number, involving covering links and Alexander invariants. As an application, we completely determine the splitting numbers of links with 9 or fewer crossings. Also, with these techniques, we either reprove or improve upon the lower bounds for splitting numbers of links computed by J. Batson and C. Seed using Khovanov homology. 1. Introduction Any link in S3 can be converted to the split union of its component knots by a sequence of crossing changes between different components. Following J. Batson and C. Seed [BS13], we define the splitting number of a link L, denoted by sp(L), as the minimal number of crossing changes in such a sequence. We present two new techniques for obtaining lower bounds for the splitting number. The first approach uses covering links, and the second method arises from the multivariable Alexander polynomial of a link. Our general covering link theorem is stated as Theorem 3.2. -

Matrix Integrals and Knot Theory 1
Matrix Integrals and Knot Theory 1 Matrix Integrals and Knot Theory Paul Zinn-Justin et Jean-Bernard Zuber math-ph/9904019 (Discr. Math. 2001) math-ph/0002020 (J.Kn.Th.Ramif. 2000) P. Z.-J. (math-ph/9910010, math-ph/0106005) J. L. Jacobsen & P. Z.-J. (math-ph/0102015, math-ph/0104009). math-ph/0303049 (J.Kn.Th.Ramif. 2004) G. Schaeffer and P. Z.-J., math-ph/0304034 P. Z.-J. and J.-B. Z., review article in preparation Carghjese, March 25, 2009 Matrix Integrals and Knot Theory 2 Courtesy of “U Rasaghiu” Matrix Integrals and Knot Theory 3 Main idea : Use combinatorial tools of Quantum Field Theory in Knot Theory Plan I Knot Theory : a few definitions II Matrix integrals and Link diagrams 1 2 4 dMeNtr( 2 M + gM ) N N matrices, N ∞ − × → RemovalsR of redundancies reproduces recent results of Sundberg & Thistlethwaite (1998) ⇒ based on Tutte (1963) III Virtual knots and links : counting and invariants Matrix Integrals and Knot Theory 4 Basics of Knot Theory a knot , links and tangles Equivalence up to isotopy Problem : Count topologically inequivalent knots, links and tangles Represent knots etc by their planar projection with minimal number of over/under-crossings Theorem Two projections represent the same knot/link iff they may be transformed into one another by a sequence of Reidemeister moves : ; ; Matrix Integrals and Knot Theory 5 Avoid redundancies by keeping only prime links (i.e. which cannot be factored) Consider the subclass of alternating knots, links and tangles, in which one meets alternatingly over- and under-crossings. For n 8 (resp. -

Knot Theory and the Alexander Polynomial
Knot Theory and the Alexander Polynomial Reagin Taylor McNeill Submitted to the Department of Mathematics of Smith College in partial fulfillment of the requirements for the degree of Bachelor of Arts with Honors Elizabeth Denne, Faculty Advisor April 15, 2008 i Acknowledgments First and foremost I would like to thank Elizabeth Denne for her guidance through this project. Her endless help and high expectations brought this project to where it stands. I would Like to thank David Cohen for his support thoughout this project and through- out my mathematical career. His humor, skepticism and advice is surely worth the $.25 fee. I would also like to thank my professors, peers, housemates, and friends, particularly Kelsey Hattam and Katy Gerecht, for supporting me throughout the year, and especially for tolerating my temporary insanity during the final weeks of writing. Contents 1 Introduction 1 2 Defining Knots and Links 3 2.1 KnotDiagramsandKnotEquivalence . ... 3 2.2 Links, Orientation, and Connected Sum . ..... 8 3 Seifert Surfaces and Knot Genus 12 3.1 SeifertSurfaces ................................. 12 3.2 Surgery ...................................... 14 3.3 Knot Genus and Factorization . 16 3.4 Linkingnumber.................................. 17 3.5 Homology ..................................... 19 3.6 TheSeifertMatrix ................................ 21 3.7 TheAlexanderPolynomial. 27 4 Resolving Trees 31 4.1 Resolving Trees and the Conway Polynomial . ..... 31 4.2 TheAlexanderPolynomial. 34 5 Algebraic and Topological Tools 36 5.1 FreeGroupsandQuotients . 36 5.2 TheFundamentalGroup. .. .. .. .. .. .. .. .. 40 ii iii 6 Knot Groups 49 6.1 TwoPresentations ................................ 49 6.2 The Fundamental Group of the Knot Complement . 54 7 The Fox Calculus and Alexander Ideals 59 7.1 TheFreeCalculus ............................... -

Maximum Knotted Switching Sequences
Maximum Knotted Switching Sequences Tom Milnor August 21, 2008 Abstract In this paper, we define the maximum knotted switching sequence length of a knot projection and investigate some of its properties. In par- ticular, we examine the effects of Reidemeister and flype moves on maxi- mum knotted switching sequences, show that every knot has projections having a full sequence, and show that all minimal alternating projections of a knot have the same length maximum knotted switching sequence. Contents 1 Introduction 2 1.1 Basic definitions . 2 1.2 Changing knot projections . 3 2 Maximum knotted switching sequences 4 2.1 Immediate bounds . 4 2.2 Not all maximal knotted switching sequences are maximum . 5 2.3 Composite knots . 5 2.4 All knots have a projection with a full sequence . 6 2.5 An infinite family of knots with projections not having full se- quences . 6 3 The effects of Reidemeister moves on the maximum knotted switching sequence length of a knot projection 7 3.1 Type I Reidemeister moves . 7 3.2 Type II Reidemeister moves . 8 3.3 Alternating knots and Type II Reidemeister moves . 9 3.4 The Conway polynomial and Type II Reidemeister moves . 13 3.5 Type III Reidemeister moves . 15 4 The maximum sequence length of minimal alternating knot pro- jections 16 4.1 Flype moves . 16 1 1 Introduction 1.1 Basic definitions A knot is a smooth embedding of S1 into R3 or S3. A knot is said to be an unknot if it is isotopic to S1 ⊂ R3. Otherwise it is knotted. -

UW Math Circle May 26Th, 2016
UW Math Circle May 26th, 2016 We think of a knot (or link) as a piece of string (or multiple pieces of string) that we can stretch and move around in space{ we just aren't allowed to cut the string. We draw a knot on piece of paper by arranging it so that there are two strands at every crossing and by indicating which strand is above the other. We say two knots are equivalent if we can arrange them so that they are the same. 1. Which of these knots do you think are equivalent? Some of these have names: the first is the unkot, the next is the trefoil, and the third is the figure eight knot. 2. Find a way to determine all the knots that have just one crossing when you draw them in the plane. Show that all of them are equivalent to an unknotted circle. The Reidemeister moves are operations we can do on a diagram of a knot to get a diagram of an equivalent knot. In fact, you can get every equivalent digram by doing Reidemeister moves, and by moving the strands around without changing the crossings. Here are the Reidemeister moves (we also include the mirror images of these moves). We want to have a way to distinguish knots and links from one another, so we want to extract some information from a digram that doesn't change when we do Reidemeister moves. Say that a crossing is postively oriented if it you can rotate it so it looks like the left hand picture, and negatively oriented if you can rotate it so it looks like the right hand picture (this depends on the orientation you give the knot/link!) For a link with two components, define the linking number to be the absolute value of the number of positively oriented crossings between the two different components of the link minus the number of negatively oriented crossings between the two different components, #positive crossings − #negative crossings divided by two. -

Knot and Link Tricolorability Danielle Brushaber Mckenzie Hennen Molly Petersen Faculty Mentor: Carolyn Otto University of Wisconsin-Eau Claire
Knot and Link Tricolorability Danielle Brushaber McKenzie Hennen Molly Petersen Faculty Mentor: Carolyn Otto University of Wisconsin-Eau Claire Problem & Importance Colorability Tables of Characteristics Theorem: For WH 51 with n twists, WH 5 is tricolorable when Knot Theory, a field of Topology, can be used to model Original Knot The unknot is not tricolorable, therefore anything that is tri- 1 colorable cannot be the unknot. The prime factors of the and understand how enzymes (called topoisomerases) work n = 3k + 1 where k ∈ N ∪ {0}. in DNA processes to untangle or repair strands of DNA. In a determinant of the knot or link provides the colorability. For human cell nucleus, the DNA is linear, so the knots can slip off example, if a knot’s determinant is 21, it is 3-colorable (tricol- orable), and 7-colorable. This is known by the theorem that Proof the end, and it is difficult to recognize what the enzymes do. Consider WH 5 where n is the number of the determinant of a knot is 0 mod n if and only if the knot 1 However, the DNA in mitochon- full positive twists. n is n-colorable. dria is circular, along with prokary- Link Colorability Det(L) Unknot/Link (n = 1...n = k) otic cells (bacteria), so the enzyme L Number Theorem: If det(L) = 0, then L is WOLOG, let WH 51 be colored in this way, processes are more noticeable in 3 3 3 1 n 1 n-colorable for all n. excluding coloring the twist component. knots in this type of DNA.