From Cytoskeletal Dynamics to Organ Asymmetry: a Non-Linear, Regulative Pathway Underlies Left-Right Patterning
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Isolated Levocardia with Situs Inversus Without Cardiac Abnormality in Fetus: Prenatal Diagnosis and Management
A L J O A T U N R I N R A E L P Case Report P L E R A Perinatal Journal 2020;28(1):48–51 I N N R A U T A L J O ©2020 Perinatal Medicine Foundation Isolated levocardia with situs inversus without cardiac abnormality in fetus: prenatal diagnosis and management Mucize Eriç ÖzdemirİD , Oya Demirci İD Department of Perinatology, Zeynep Kamil Maternity and Children’s Diseases Training and Research Hospital., Health Sciences University, Istanbul, Turkey Abstract Özet: Kardiyak anomalisi olmayan fetüste situs inversuslu izole levokardi: Prenatal tan› ve yönetim Objective: Isolated levocardia is a situs abnormality that the heart is Amaç: ‹zole levokardi, kalbin normal levo pozisyonunda oldu¤u in the normal levo position, but the abdominal viscera are in dextro fakat abdominal iç organlar›n dekstro pozisyonunda oldu¤u bir si- position. Most cases are accompanied by structural heart anomalies. tus anomalisidir. Ço¤u olguda yap›sal kalp anomalileri de efllik et- In this case, we aimed to present a fetus with isolated levocardia with- mektedir. Çal›flmam›zda, kardiyak anomalisi olmayan izole levo- out cardiac abnormality. kardili bir fetüsü sunmay› amaçlad›k. Case: The mother was referred to our clinic with a suspicion of fetal Olgu: Olgumuz, 22. gebelik haftas›nda fetal dekstrokardi flüphe- dextrocardia at 22 weeks of gestation. When detailed examination was siyle klini¤imize sevk edildi. Planlanan detayl› ultrason muayene- planned by ultrasonography isolated levocardia was detected in fetus. sinde, fetüste izole levokardi tespit edildi. Fetal ekokardiyografide There were no cardiac abnormalities in fetal echocardiography. Fetus hiçbir kardiyak anomali görülmedi. -

From the Left to the Right a Tale of Asymmetries, Environments, and Hippocampal Development
From the left to the right A tale of asymmetries, environments, and hippocampal development by Matthew Case June, 2018 A thesis presented to the Graduate School of the Institute of Science and Technology Austria, Klosterneuburg, Austria in partial fulfilment of the requirements for the degree of Doctor of Philosophy The dissertation of Matthew Case, titled ‘From the left to the right: A tale of asymmetries, environments, and hippocampal development’, is approved by: Supervisor: [Name of Supervisor], IST Austria, Klosterneuburg, Austria Signature: Committee Member: [Name of Committee Member], IST Austria, Klosterneuburg, Austria Signature: Committee Member: [Name of Committee Member], [Institute], [City], [Country] Signature: Exam Chair: [Name of Exam Chair], IST Austria, Klosterneuburg, Austria Signature: © by Matthew Case, June, 2018 All Rights Reserved I hereby declare that this dissertation is my own work and that it does not contain other people’s work without this being so stated; this thesis does not contain my previous work without this being stated, and the bibliography contains all the literature that I used in writing the dissertation. I declare that this is a true copy of my thesis, including any final revisions, as approved by my thesis committee, and that this thesis has not been submitted for a higher degree to any other university or institution. I certify that any republication of materials presented in this thesis has been approved by the relevant publishers and co-authors. Signature: _______________________ [Matthew J Case] June 27, 2018 “Symmetry is what we see at a glance; based on the fact that there is no reason for any difference...” ― Blaise Pascal, Pensées Dedication I'd like to offer a joint dedication for this thesis. -

Asymmetry Inside the Symmetry: Mechanisms and Disorders of Laterality
Asymmetry Inside The Symmetry: Mechanisms and Disorders Of Laterality Yasemin ALANAY1, Ersin GÜMÜŞ2, Miray SEKKİN2, Sebile GÜLER2, A. Nur ÇAKAR3 Ankara-Turkey Asy mmetry of the left-right (L-R) axis is a feature of vertebrates that is apparent in the viscera and vas- culature. An understanding of L-R patterning in human development is aided by familiarity with key ev ents and important pathways in mammalian embryogenesis.The induction of L-R asymmetry follows the establishment of the anteroposterior (A-P or rostrocaudal) and dorsoventral (D-V) axes and re- quires the pre-existing midline symmetry to be broken before sidedness is induced via a series of complex, coordinated molecular signaling events. In this review, our aim is to describe the molecular background of this embryological process, and giv e brief knowledge concerning its clinical conse- quences. (Gynecol Obstet Reprod Med 2006; 12:000-000) Key Words: Heterotaxy, Laterality, Situs anomalies Many living creatures in nature, from Ascidians to man, T here are also many l ess defin ed asymmetries that hav e exhibit symmetry which can be described as correspondence been the focus of many studies to date. Human hand prefe- in size, shape and relative position of congruent components rence and the functional asymmetry of the brain hemispheres are still attractive research areas, and the basics of genetic about a plane or axis, as a feature of their external body plan. 1 Most animals possess symmetrical body plans such as; sphe- mechanisms are still controversial. Cell mediated immune 2 rical, radial, chiral, bilateral and pseudo-bilateral. In har- hypersensitivity is stronger on the left side of the body and mony with this, possession of a symmetrical body plan is the left side of the scrotum descends lower than the right in 3 one of the conspicuous morphological characteristics of ver- correlation with handedness. -

Imaging Evaluation of the Heterotaxy Syndrome
Gastroenterology & Hepatology: Open Access Review Article Open Access Imaging evaluation of the heterotaxy syndrome Abstract Volume 10 Issue 2 - 2019 Situs ambiguus, or heterotaxy, is a rare syndrome characterized by an abnormal José Maximiliano Garófano-Jerez,1,2,3 Elena arrangement of the internal organs in the chest and abdomen and is usually associated Benedicto-Hernández,2 Juan de Dios with congenital heart diseases. Despite its variable presentation, heterotaxy syndrome 4 can be classified into heterotaxy with polysplenia or left isomerism, and heterotaxy López-González Gila, Juan de Dios López- 1,2 with asplenia or right isomerism. The different imaging techniques reveal the González Garrido characteristic imaging findings that allow an accurate description of each patient’s 1Departamento de Radiología y Medicina Física, Spain 2 specific anatomy. Evaluation of the position of the following structures relative to Facultad de Medicina, Universidad de Granada, Spain 3 midline is crucial: atria, cardiac apex, venous drainage, aorta, stomach, liver and Servicio de Radiodiagnóstico, Hospital Universitario San Cecilio de Granada, Spain gallbladder, as well as the presence or absence of intestinal malrotation, number of 4Servicio de Nefrología del Hospital Universitario Virgen de las spleens, and presence of tri- or bilobed lungs. Nieves de Granada, Spain We present a case report of a 42-year-old man with dextrocardia underwent laparoscopic cholecystectomy for cholelithiasis. Subsequently, a complete resection Correspondence: Juan de Dios López-González Garrido, of the common bile duct required reconstruction of the biliary tract. During six years Profesor Titular de Universidad. Departamento de Radiología y Medicina Física de la Facultad de Medicina. Avda. -
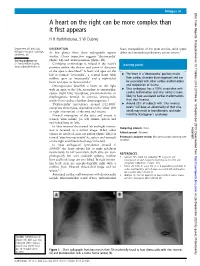
A Heart on the Right Can Be More Complex Than It First Appears
Images in... BMJ Case Reports: first published as 10.1136/bcr-2013-201046 on 19 September 2013. Downloaded from A heart on the right can be more complex than it first appears H R Haththotuwa, S W Dubrey Department of Cardiology, DESCRIPTION heart, transposition of the great arteries, atrial septal Hillingdon Hospital, Uxbridge, At first glance these chest radiographs appear defect and anomalous pulmonary venous return.3 Middlesex, UK similar. Closer inspection suggests ‘dextrocardia’ Correspondence to (figure 1A) and ‘dextroposition’ (figure 1B). ’ Dr Simon William Dubrey, Confusing terminology is helped if the heart s Learning points [email protected] position within the thorax and point of direction of the apex is described.1 A heart and apex on the ▸ ‘ ’ left is termed ‘levocardia’, a central heart with The heart in a dextrocardia position results midline apex as ‘mesocardia’ and a right-sided from cardiac chamber disarrangement and can heart and apex as ‘dextrocardia’. be associated with other cardiac malformations Dextroposition describes a heart on the right and malposition of viscera. ▸ with an apex to the left, secondary to extracardiac Situs ambiguous has a 100% association with causes (right lung hypoplasia, pneumonectomy or cardiac malformation and situs solitus is more diaphragmatic hernia). In contrast, dextrocardia likely to have associated cardiac malformations results from cardiac chamber disarrangement.2 than situs inversus. 2 ▸ ‘ ‘Dextrocardia’ (prevalence around 1/12 000) Around 20% of subjects with situs inversus ’ comprises three types, depending on the ‘situs’ (left totalis will have an abnormality of their cilia, or right orientation) of the atria and viscera. which may result in bronchiectasis and male ’ Normal orientation of the atria and viscera is infertility (Kartagener s syndrome). -

Brain Structural and Functional Asymmetry in Human Situs Inversus Totalis
Edinburgh Research Explorer Brain structural and functional asymmetry in human situs inversus totalis Citation for published version: Vingerhoets, G, Li, X, Hou, L, Bogaert, S, Verhelst, H, Gerrits, R, Siugzdaite, R & Roberts, N 2018, 'Brain structural and functional asymmetry in human situs inversus totalis' Brain Structure and Function. DOI: 10.1007/s00429-017-1598-5 Digital Object Identifier (DOI): 10.1007/s00429-017-1598-5 Link: Link to publication record in Edinburgh Research Explorer Document Version: Peer reviewed version Published In: Brain Structure and Function General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 24. Jul. 2019 Brain Structure and Function Brain Structural and Functional Asymmetry in Human Situs Inversus Totalis --Manuscript Draft-- Manuscript Number: BSAF-D-17-00474R1 Full Title: Brain Structural and Functional Asymmetry in Human Situs Inversus Totalis Article Type: Original Article Keywords: brain -

Visceral Situs, Heart Position, and Aortic Arch Position 69
Visceral Situs, Heart Position, 7 and Aortic Arch Position The second step in interpretation of a chest radiograph and (6) the azygos vein. The basic concepts regarding vis- obtained in a new patient is to ascertain visceral situs, the ceral situs is discussed in detail in Chapter 2. heart position, and the position of the aortic arch relative In situs solitus, the gastric air bubble is on the left side, to the trachea. For example, the radiographic report may and the larger lobe of the liver is on the right side (Fig. 7.2). start with this sentence: “The radiograph shows situs solitus, The splenic tip can often be identified when the stomach levocardia and a left aortic arch.” and adjacent bowel loops are filled with air. When the bronchial air column can be traced, an asymmetric bronchial branching pattern with a short right and a long left main bronchus can be appreciated on the frontal radi- ■ Visceral Situs ograph. Normally the left main bronchus is 1.5 to 2 times longer than the right main bronchus. At the pulmonary Visceral situs refers to the pattern of arrangement of the hilum, the left pulmonary artery is seen slightly higher body organs relative to the midline. There are four types than the right pulmonary artery. The left pulmonary of visceral situs: situs solitus, situs inversus, heterotaxy artery is seen above the left upper lobe bronchus, with thoracic right isomerism, and heterotaxy with whereas the right pulmonary artery (in fact, its descend- thoracic left isomerism (Fig. 7.1).1–4 Visceral heterotaxy ing branch) is seen below the right upper lobe bronchus. -

Increased Prevalence of Left-Handedness in Hemifacial Microsomia
ORIGINAL ARTICLE Increased Prevalence of Left-Handedness in Hemifacial Microsomia Gary F. Rogers, MD, JD, MBA, MPH,* Stephen R. Sullivan, MD,* John B. Mulliken, MD,* Arin K. Greene, MD, MMSc,* and Albert K. Oh, MDÞ he body plan for humans is essentially symmetric around the Abstract: Ten percent of people are left handed, but a higher Tmidaxis. Nevertheless, asymmetric arrangement of organs in the frequency has been associated with certain craniofacial malforma- thoracoabdominal cavities indicates that cellular functions become tions, such as cleft lip and unilateral coronal synostosis. The purpose unequal early in development. Cellular symmetry persists through- of this study was to determine the frequency of left-handedness in out the gastrula stage and, thereafter, differential cascades of gene patients with hemifacial microsomia (HFM). Patients with HFM expression break the otherwise symmetrical internal structure.1 were identified in our craniofacial database. Normal controls were Animal models have provided some insight into the molecular and recruited by local pediatricians. Data gathered included age, sex, developmental mechanisms by which right-left symmetry is 1,2 and handedness (determined by writing and/or drawing); the orbit, altered. Nevertheless, investigators are still unclear why these mandible, ear, nerve, and soft tissue (OMENS)Yplus score and side structural asymmetries occur in a nonrandom, or unequal, distribu- tion. Nonrandom laterality is seen in the human brain, face, heart, of involvement were tabulated for patients with HFM. Hand pre- 2 great vessels, lungs, liver, gallbladder, biliary tract, gastrointestinal ference was compared between the groups using # analysis; pos- tract, spleen, and male genitalia.3,4 All but 0.01% of humans exhibit sible correlations were analyzed between handedness and age, the same asymmetric arrangement of their internal organs (situs or sex, the OMENS score, extracraniofacial findings, and side of in- situs solitus).5,6 Nevertheless, in extremely rare instances, the volvement. -

Atypical Brain Asymmetry in Human Situs Inversus: Gut Feeling Or Real Evidence?
S S symmetry Review Atypical Brain Asymmetry in Human Situs Inversus: Gut Feeling or Real Evidence? Guy Vingerhoets * , Robin Gerrits and Helena Verhelst Department of Experimental Psychology, Ghent University, 9000 Ghent, Belgium; [email protected] (R.G.); [email protected] (H.V.) * Correspondence: [email protected] Abstract: The alignment of visceral and brain asymmetry observed in some vertebrate species raises the question of whether this association also exists in humans. While the visceral and brain systems may have developed asymmetry for different reasons, basic visceral left–right differentiation mechanisms could have been duplicated to establish brain asymmetry. We describe the main phenotypical anomalies and the general mechanism of left–right differentiation of vertebrate visceral and brain laterality. Next, we systematically review the available human studies that explored the prevalence of atypical behavioral and brain asymmetry in visceral situs anomalies, which almost exclusively involved participants with the mirrored visceral organization (situs inversus). The data show no direct link between human visceral and brain functional laterality as most participants with situs inversus show the typical population bias for handedness and brain functional asymmetry, although an increased prevalence of functional crowding may be present. At the same time, several independent studies present evidence for a possible relation between situs inversus and the gross morphological asymmetry of the brain torque with potential differences between subtypes of situs inversus with ciliary and non-ciliary etiologies. Citation: Vingerhoets, G.; Gerrits, R.; Verhelst, H. Atypical Brain Keywords: situs inversus; heterotaxy; brain asymmetry; visceral asymmetry; vertebrate asymmetry; Asymmetry in Human Situs Inversus: human laterality; left-right differentiation; brain torque; ciliopathy Gut Feeling or Real Evidence?. -

Factors Influencing Age at Diagnosis of Primary Ciliary Dyskinesia in European Children
Eur Respir J 2010; 36: 1248–1258 DOI: 10.1183/09031936.00001010 CopyrightßERS 2010 ERS TASK FORCE REPORT Factors influencing age at diagnosis of primary ciliary dyskinesia in European children C.E. Kuehni*, T. Frischer#, M-P.F. Strippoli*, E. Maurer*, A. Bush", K.G. Nielsen+, A. Escribano1, J.S.A. Lucase, P. Yiallouros**, H. Omran##, E. Eber"", C. O’Callaghan++, D. Snijders11 and A. Barbato11 for the ERS Task Force on Primary Ciliary Dyskinesia in Childrenee ABSTRACT: Primary ciliary dyskinesia (PCD) is a hereditary disorder of mucociliary clearance AFFILIATIONS causing chronic upper and lower airways disease. We determined the number of patients with *Institute of Social and Preventive Medicine (ISPM), University of Berne, Berne, diagnosed PCD across Europe, described age at diagnosis and determined risk factors for late Switzerland. diagnosis. #Universita¨tsklinik fu¨r Kinder- und Jugendheilkunde, Vienna, Centres treating children with PCD in Europe answered questionnaires and provided "" Dept of Paediatrics and Adolescence anonymous patient lists. Medicine, Medical University of Graz, Graz, In total, 223 centres from 26 countries reported 1,009 patients aged ,20 yrs. Reported cases Austria. "Royal Brompton Hospital, London, per million children (for 5–14 yr olds) were highest in Cyprus (111), Switzerland (47) and Denmark e Division of Infection, Inflammation and (46). Overall, 57% were males and 48% had situs inversus. Median age at diagnosis was 5.3 yrs, Immunity, University of Southampton lower in children with situs inversus (3.5 versus 5.8 yrs; p,0.001) and in children treated in large School of Medicine, Southampton, ++Division of Child Health, Dept of centres (4.1 versus 4.8 yrs; p50.002). -

UCLA Previously Published Works
UCLA UCLA Previously Published Works Title Genetic steps to organ laterality in zebrafish. Permalink https://escholarship.org/uc/item/7sx543rc Journal Comparative and functional genomics, 2(2) ISSN 1531-6912 Authors Chen, JN van Bebber, F Goldstein, AM et al. Publication Date 2001 DOI 10.1002/cfg.74 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Comparative and Functional Genomics Comp Funct Genom 2001; 2: 60–68. DOI: 10.1002/cfg.74 Research Article Genetic steps to organ laterality in zebrafish Jau-Nian Chen1, Frauke van Bebber2, Allan M. Goldstein1, Fabrizio C. Serluca1, Donald Jackson1, Sarah Childs1, George Serbedzija1, Kerri S. Warren1, John D. Mably1, Per Lindahl1, Alan Mayer1, Pascal Haffter2 and Mark C. Fishman1* 1 Cardiovascular Research Center, Massachusetts General Hospital, 149 13th Street, Charlestown, MA 02129, and Department of Medicine, Harvard Medical School, Boston, MA 02115, USA 2 Max-Planck-Institut fu¨r Entwicklungsbiologie, Abteilung Genetik, Spemannstrasse 35, 72076, Tu¨bingen, Germany *Correspondence to: Abstract M. C. Fishman, Cardiovascular All internal organs are asymmetric along the left–right axis. Here we report a genetic Research Center, Massachusetts screen to discover mutations which perturb organ laterality. Our particular focus is upon General Hospital, 149 13th whether, and how, organs are linked to each other as they achieve their laterally Street, Charlestown, MA 02129, asymmetric positions. We generated mutations by ENU mutagenesis and examined F3 USA and Department of Medicine, Harvard Medical progeny using a cocktail of probes that reveal early primordia of heart, gut, liver and School, Boston, MA 02115, USA. pancreas. From the 750 genomes examined, we isolated seven recessive mutations which E-mail: affect the earliest left–right positioning of one or all of the organs. -

Handedness and Situs Inversus in Primary Ciliary Dyskinesia I
Received 20 February 2004 Accepted 5 August 2004 Published online 9 December 2004 Handedness and situs inversus in primary ciliary dyskinesia I. C. McManus1Ã, N. Martin1, G. F. Stubbings1, E. M. K. Chung2 and H. M. Mitchison2 1Department of Psychology, University College London, Gower Street, London WC1E 6BT, UK 2Department of Paediatrics and Child Health, University College London, Gower Street, London WC1E 6BT, UK ...The limbs on the right side are stronger. [The] cause may be ...[that] ...motion, and abilities of moving, are somewhat holpen from the liver, which lieth on the right side. (Sir Francis Bacon, Sylva sylvarum (1627).) Fifty per cent of people with primary ciliary dyskinesia (PCD) (also known as immotile cilia syndrome or Siewert–Kartagener syndrome) have situs inversus, which is thought to result from absent nodal ciliary rotation and failure of normal symmetry breaking. In a study of 88 people with PCD, only 15.2% of 46 indi- viduals with situs inversus, and 14.3% of 42 individuals with situs solitus, were left handed. Because cerebral lateralization is therefore still present, the nodal cilia cannot be the primary mechanism responsible for sym- metry breaking in the vertebrate body. Intriguingly, one behavioural lateralization, wearing a wrist-watch on the right wrist, did correlate with situs inversus. Keywords: handedness; lateralization; situs inversus; primary ciliary dyskinesia; Siewert–Kartagener syndrome 1. INTRODUCTION from perfect but nevertheless can be explained by a Humans, like other vertebrates, mostly have their heart on straightforward genetic model (McManus 1985, 1999; the left side, and there is a secondary asymmetry of other Annett & Alexander 1996).