Diagnosis, Monitoring, and Treatment of Primary Ciliary Dyskinesia: PCD Foundation Consensus Recommendations Based on State of the Art Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Jersey Chapter American College of Physicians
NEW JERSEY CHAPTER AMERICAN COLLEGE OF PHYSICIANS ASSOCIATES ABSTRACT COMPETITION 2015 SUBMISSIONS 2015 Resident/Fellow Abstracts 1 1. ID CATEGORY NAME ADDITIONAL PROGRAM ABSTRACT AUTHORS 2. 295 Clinical Abed, Kareem Viren Vankawala MD Atlanticare Intrapulmonary Arteriovenous Malformation causing Recurrent Cerebral Emboli Vignette FACC; Qi Sun MD Regional Medical Ischemic strokes are mainly due to cardioembolic occlusion of small vessels, as well as large vessel thromboemboli. We describe a Center case of intrapulmonary A-V shunt as the etiology of an acute ischemic event. A 63 year old male with a past history of (Dominik supraventricular tachycardia and recurrent deep vein thrombosis; who has been non-compliant on Rivaroxaban, presents with Zampino) pleuritic chest pain and was found to have a right lower lobe pulmonary embolus. The deep vein thrombosis and pulmonary embolus were not significant enough to warrant ultrasound-enhanced thrombolysis by Ekosonic EndoWave Infusion Catheter System, and the patient was subsequently restarted on Rivaroxaban and discharged. The patient presented five days later with left arm tightness and was found to have multiple areas of punctuate infarction of both cerebellar hemispheres, more confluent within the right frontal lobe. Of note he was compliant at this time with Rivaroxaban. The patient was started on unfractionated heparin drip and subsequently admitted. On admission, his vital signs showed a blood pressure of 138/93, heart rate 65 bpm, and respiratory rate 16. Cardiopulmonary examination revealed regular rate and rhythm, without murmurs, rubs or gallops and his lungs were clear to auscultation. Neurologic examination revealed intact cranial nerves, preserved strength in all extremities, mild dysmetria in the left upper extremity and an NIH score of 1. -

Isolated Levocardia with Situs Inversus Without Cardiac Abnormality in Fetus: Prenatal Diagnosis and Management
A L J O A T U N R I N R A E L P Case Report P L E R A Perinatal Journal 2020;28(1):48–51 I N N R A U T A L J O ©2020 Perinatal Medicine Foundation Isolated levocardia with situs inversus without cardiac abnormality in fetus: prenatal diagnosis and management Mucize Eriç ÖzdemirİD , Oya Demirci İD Department of Perinatology, Zeynep Kamil Maternity and Children’s Diseases Training and Research Hospital., Health Sciences University, Istanbul, Turkey Abstract Özet: Kardiyak anomalisi olmayan fetüste situs inversuslu izole levokardi: Prenatal tan› ve yönetim Objective: Isolated levocardia is a situs abnormality that the heart is Amaç: ‹zole levokardi, kalbin normal levo pozisyonunda oldu¤u in the normal levo position, but the abdominal viscera are in dextro fakat abdominal iç organlar›n dekstro pozisyonunda oldu¤u bir si- position. Most cases are accompanied by structural heart anomalies. tus anomalisidir. Ço¤u olguda yap›sal kalp anomalileri de efllik et- In this case, we aimed to present a fetus with isolated levocardia with- mektedir. Çal›flmam›zda, kardiyak anomalisi olmayan izole levo- out cardiac abnormality. kardili bir fetüsü sunmay› amaçlad›k. Case: The mother was referred to our clinic with a suspicion of fetal Olgu: Olgumuz, 22. gebelik haftas›nda fetal dekstrokardi flüphe- dextrocardia at 22 weeks of gestation. When detailed examination was siyle klini¤imize sevk edildi. Planlanan detayl› ultrason muayene- planned by ultrasonography isolated levocardia was detected in fetus. sinde, fetüste izole levokardi tespit edildi. Fetal ekokardiyografide There were no cardiac abnormalities in fetal echocardiography. Fetus hiçbir kardiyak anomali görülmedi. -

From the Left to the Right a Tale of Asymmetries, Environments, and Hippocampal Development
From the left to the right A tale of asymmetries, environments, and hippocampal development by Matthew Case June, 2018 A thesis presented to the Graduate School of the Institute of Science and Technology Austria, Klosterneuburg, Austria in partial fulfilment of the requirements for the degree of Doctor of Philosophy The dissertation of Matthew Case, titled ‘From the left to the right: A tale of asymmetries, environments, and hippocampal development’, is approved by: Supervisor: [Name of Supervisor], IST Austria, Klosterneuburg, Austria Signature: Committee Member: [Name of Committee Member], IST Austria, Klosterneuburg, Austria Signature: Committee Member: [Name of Committee Member], [Institute], [City], [Country] Signature: Exam Chair: [Name of Exam Chair], IST Austria, Klosterneuburg, Austria Signature: © by Matthew Case, June, 2018 All Rights Reserved I hereby declare that this dissertation is my own work and that it does not contain other people’s work without this being so stated; this thesis does not contain my previous work without this being stated, and the bibliography contains all the literature that I used in writing the dissertation. I declare that this is a true copy of my thesis, including any final revisions, as approved by my thesis committee, and that this thesis has not been submitted for a higher degree to any other university or institution. I certify that any republication of materials presented in this thesis has been approved by the relevant publishers and co-authors. Signature: _______________________ [Matthew J Case] June 27, 2018 “Symmetry is what we see at a glance; based on the fact that there is no reason for any difference...” ― Blaise Pascal, Pensées Dedication I'd like to offer a joint dedication for this thesis. -

Supermicar Data Entry Instructions, 2007 363 Pp. Pdf Icon[PDF
SUPERMICAR TABLE OF CONTENTS Chapter I - Introduction to SuperMICAR ........................................... 1 A. History and Background .............................................. 1 Chapter II – The Death Certificate ..................................................... 3 Exercise 1 – Reading Death Certificate ........................... 7 Chapter III Basic Data Entry Instructions ....................................... 12 A. Creating a SuperMICAR File ....................................... 14 B. Entering and Saving Certificate Data........................... 18 C. Adding Certificates using SuperMICAR....................... 19 1. Opening a file........................................................ 19 2. Certificate.............................................................. 19 3. Sex........................................................................ 20 4. Date of Death........................................................ 20 5. Age: Number of Units ........................................... 20 6. Age: Unit............................................................... 20 7. Part I, Cause of Death .......................................... 21 8. Duration ................................................................ 22 9. Part II, Cause of Death ......................................... 22 10. Was Autopsy Performed....................................... 23 11. Were Autopsy Findings Available ......................... 23 12. Tobacco................................................................ 24 13. Pregnancy............................................................ -

2018 Southern Regional Meeting
Abstracts J Investig Med: first published as 10.1136/jim-2017-000697.1 on 1 February 2018. Downloaded from Cardiovascular club I Purpose of study Coronary artery disease (C.A.D.) is one of the highest causes of death in the world. The purpose of this 11:00 AM study is to compare Puerto Rico (P.R.), Hispanic, U.S.A. coun- try, with the U.S.A., in coronary artery disease. Thursday, February 22, 2018 Methods used Compare a population of Hispanics with the high LDL levels with normal total cholesterol and HDL in P. R. and the U.S.A. The study population was 1000 patients. 1 A POSSIBLE ROLE FOR GENETICS IN CARDIOVASCULAR The U.S.A. health statistics and P.R. Department of Health DISEASE AMONG THE ACADIANS was used for comparison. AE Tedesco*, Z Haq, KL Di Losa, MJ Ali, P Gregory. LSUHSC – New Orleans, New Orleans, Summary of results Studying the lipid profile of Puerto Rico LA population, we found that the mean value of LDL lipoprotein is high (±104 mg/dl) with similar cholesterol and HDL levels 10.1136/jim-2017-000697.1 in both societies; still the coronary disease (CAD) incidence is lower than the U.S.A. (20%–30%). Investigators from the U.P. Purpose of study It is well documented that the Louisiana Aca- R. reported the genetic admixture of this Hispanic population. ‘ ’ dians ( Cajuns ) experience a disproportionate risk for some They reported the admixture consisted of 3 genes called pro- genetic diseases due to a genetic founder effect.Furthermore, cer- tective against C.A.D. -

Asymmetry Inside the Symmetry: Mechanisms and Disorders of Laterality
Asymmetry Inside The Symmetry: Mechanisms and Disorders Of Laterality Yasemin ALANAY1, Ersin GÜMÜŞ2, Miray SEKKİN2, Sebile GÜLER2, A. Nur ÇAKAR3 Ankara-Turkey Asy mmetry of the left-right (L-R) axis is a feature of vertebrates that is apparent in the viscera and vas- culature. An understanding of L-R patterning in human development is aided by familiarity with key ev ents and important pathways in mammalian embryogenesis.The induction of L-R asymmetry follows the establishment of the anteroposterior (A-P or rostrocaudal) and dorsoventral (D-V) axes and re- quires the pre-existing midline symmetry to be broken before sidedness is induced via a series of complex, coordinated molecular signaling events. In this review, our aim is to describe the molecular background of this embryological process, and giv e brief knowledge concerning its clinical conse- quences. (Gynecol Obstet Reprod Med 2006; 12:000-000) Key Words: Heterotaxy, Laterality, Situs anomalies Many living creatures in nature, from Ascidians to man, T here are also many l ess defin ed asymmetries that hav e exhibit symmetry which can be described as correspondence been the focus of many studies to date. Human hand prefe- in size, shape and relative position of congruent components rence and the functional asymmetry of the brain hemispheres are still attractive research areas, and the basics of genetic about a plane or axis, as a feature of their external body plan. 1 Most animals possess symmetrical body plans such as; sphe- mechanisms are still controversial. Cell mediated immune 2 rical, radial, chiral, bilateral and pseudo-bilateral. In har- hypersensitivity is stronger on the left side of the body and mony with this, possession of a symmetrical body plan is the left side of the scrotum descends lower than the right in 3 one of the conspicuous morphological characteristics of ver- correlation with handedness. -

Heterotaxy: Associated Conditions and Hospital- Based Prevalence in Newborns Angela E
May/June 2000. Vol. 2 . No. 3 Heterotaxy: Associated conditions and hospital- based prevalence in newborns Angela E. Lin, MD',~,*, Baruch S. Ticho, MD, phD3j4,Kara Houde, MA1,Marie-Noel Westgate, ME^', and Lewis B. Holmes, MD',~,~ Purpose: To provide insight into the possible etiology and prevalence of heterotaxy, we studied conditions associated with heterotaxy in a consecutive hospital population of newborns. Methods: From 1972 to March, 1999 (except February 16, 1972 to December 31, 1978), 58 cases of heterotaxy were ascertained from a cohort of 201,084 births in the ongoing Active Malformation Surveillance Program at the Brigham and Women's Hospital. This registry includes livebirths, stillbirths, and elective abortions. Prevalence among nontransfers (i.e., patients whose mothers had planned delivery at this hospital) was calculated as approximately 1per 10,000 total births (20 of 201,084). Results: We analyzed a total of 58 patients consisting of 20 (34%) nontransfers and 38 (66%) transfers. Patients were categorized by spleen status as having asplenia (7 nontransfers, 25 total), polysplenia (8, 20), right spleen (4, ll),normal left (0, I), and unknown (1, 0). Among the 20 nontransfer and 59 total heterotaxy patients, the following associated medical conditions were present: chromosome abnormality (1nontransfer, 2 total), suspected Mendelian or chromosome microdeletion disorder (1nontransfer, 6 total), and maternal insulin- dependent diabetes mellitus (1 nontransfer, 2 total). There were 6 twins (1 member each from 6 twin pairs including 1dizygous, 4 monozygous, 1conjoined; 2 were nontransfers). An associated condition occurred in 5 (25%) nontransfer and 16 (28%) total patients, or among 10 of 53 singleton births (19%). -

Imaging Evaluation of the Heterotaxy Syndrome
Gastroenterology & Hepatology: Open Access Review Article Open Access Imaging evaluation of the heterotaxy syndrome Abstract Volume 10 Issue 2 - 2019 Situs ambiguus, or heterotaxy, is a rare syndrome characterized by an abnormal José Maximiliano Garófano-Jerez,1,2,3 Elena arrangement of the internal organs in the chest and abdomen and is usually associated Benedicto-Hernández,2 Juan de Dios with congenital heart diseases. Despite its variable presentation, heterotaxy syndrome 4 can be classified into heterotaxy with polysplenia or left isomerism, and heterotaxy López-González Gila, Juan de Dios López- 1,2 with asplenia or right isomerism. The different imaging techniques reveal the González Garrido characteristic imaging findings that allow an accurate description of each patient’s 1Departamento de Radiología y Medicina Física, Spain 2 specific anatomy. Evaluation of the position of the following structures relative to Facultad de Medicina, Universidad de Granada, Spain 3 midline is crucial: atria, cardiac apex, venous drainage, aorta, stomach, liver and Servicio de Radiodiagnóstico, Hospital Universitario San Cecilio de Granada, Spain gallbladder, as well as the presence or absence of intestinal malrotation, number of 4Servicio de Nefrología del Hospital Universitario Virgen de las spleens, and presence of tri- or bilobed lungs. Nieves de Granada, Spain We present a case report of a 42-year-old man with dextrocardia underwent laparoscopic cholecystectomy for cholelithiasis. Subsequently, a complete resection Correspondence: Juan de Dios López-González Garrido, of the common bile duct required reconstruction of the biliary tract. During six years Profesor Titular de Universidad. Departamento de Radiología y Medicina Física de la Facultad de Medicina. Avda. -

PG Course Syllabus – FINAL
postgraduate course Atlanta,Georgia october 23, 2014 1 2 Table of Contents MODULE 1: LIVER PRIMARY SCLEROSING CHOLANGITIS – Dennis Black MD ................................................................. 13 THE JAUNDICED INFANT ‐ Saul Karpen MD ....................................................................................... 25 ACUTE LIVER FAILURE – Estella Alonso MD ....................................................................................... 43 MODULE 2: ENDOSCOPY THE DREADED WAKE‐UP CALL (PART A) – Mercedes Martinez MD .................................................. 55 THE DREADED WAKE‐UP CALL (PART B) – Lee Bass MD .................................................................... 67 ENDOSCOPIC INTERVENTIONS FOR BILIARY TRACT DISEASE – Victor Fox MD .................................. 75 MODULE 3: GI POTPOURRI EXTRAESOPHAGEAL MANIFESTATIONS OF GER – Benjamin Gold MD .............................................. 86 EoE: PPI, EGD AND WHAT TO EAT: ALPHABET DISTRESS – Sandeep Gupta MD ............................... 105 SNEW TRICK AND TREATMENTS FOR MOTILITY DISORDERS – Carlo Di Lorenzo MD ........................ 116 DIAGNOSIS AND MANAGEMENT OF CONSTIPATION – Manu Sood MD ........................................... 130 MODULE 4: NUTRITION DIET AND THE MICROBIOME – Robert Baldassano MD .................................................................... 141 FODMAP: NAVIGATING THIS NOVEL DIET – Bruno Chumpitazi MD .................................................. 152 NUTRITION EIN TH CHILD WITH NEUROLOGICAL DISABILITIES -

Asplenia and Polysplenia Syndromes with Abnormalities of Lateralisation in a Sibship
J Med Genet: first published as 10.1136/jmg.18.4.301 on 1 August 1981. Downloaded from Journal of Medical Genetics, 1981, 18, 301-302 Asplenia and polysplenia syndromes with abnormalities of lateralisation in a sibship J ZLOTOGORA AND E ELIAN From the Department ofPediatrics, Hasharon Hospital, Petah-Tiqva, and Tel-Aviv University Medical School, Israel SUMMARY In the family presented here the first child had asplenia syndrome with cor biloculare' transposition of the great vessels, pulmonary stenosis, and anomalous pulmonary venous drainage' Another sib had situs inversus with polysplenia syndrome, including very similar cardiovascular defects and biliary atresia. The possibility that these two syndromes, namely asplenia and poly- splenia, are different manifestations of a similar defect in the normal asymmetrical development of internal organs is discussed. Some degree of lateral asymmetry is one of the age and weighed 2060 g. At the age of 5 days she was characteristics of the internal human body con- noted to be cyanotic and a grade 3/6 systolic murmur figuration. A mirror image of the asymmetrical was heard along the left sternal border. At cardiac organs is found in situs inversus, while in the asplenia catheterisation, transposition of the great vessels with and polysplenia syndromes there is a striking ten- double outlet of the right ventricle and pulmonary dency for internal symmetry. In the asplenia stenosis were demonstrated. The child failed to copyright. (Ivemark) syndrome the tendency is for the left lung, thrive and died at 3 months of age following an atrium, and left lobe of the liver to resemble the episode of septicaemia. -
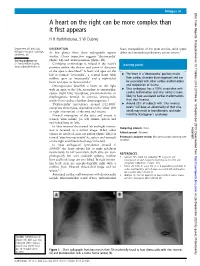
A Heart on the Right Can Be More Complex Than It First Appears
Images in... BMJ Case Reports: first published as 10.1136/bcr-2013-201046 on 19 September 2013. Downloaded from A heart on the right can be more complex than it first appears H R Haththotuwa, S W Dubrey Department of Cardiology, DESCRIPTION heart, transposition of the great arteries, atrial septal Hillingdon Hospital, Uxbridge, At first glance these chest radiographs appear defect and anomalous pulmonary venous return.3 Middlesex, UK similar. Closer inspection suggests ‘dextrocardia’ Correspondence to (figure 1A) and ‘dextroposition’ (figure 1B). ’ Dr Simon William Dubrey, Confusing terminology is helped if the heart s Learning points [email protected] position within the thorax and point of direction of the apex is described.1 A heart and apex on the ▸ ‘ ’ left is termed ‘levocardia’, a central heart with The heart in a dextrocardia position results midline apex as ‘mesocardia’ and a right-sided from cardiac chamber disarrangement and can heart and apex as ‘dextrocardia’. be associated with other cardiac malformations Dextroposition describes a heart on the right and malposition of viscera. ▸ with an apex to the left, secondary to extracardiac Situs ambiguous has a 100% association with causes (right lung hypoplasia, pneumonectomy or cardiac malformation and situs solitus is more diaphragmatic hernia). In contrast, dextrocardia likely to have associated cardiac malformations results from cardiac chamber disarrangement.2 than situs inversus. 2 ▸ ‘ ‘Dextrocardia’ (prevalence around 1/12 000) Around 20% of subjects with situs inversus ’ comprises three types, depending on the ‘situs’ (left totalis will have an abnormality of their cilia, or right orientation) of the atria and viscera. which may result in bronchiectasis and male ’ Normal orientation of the atria and viscera is infertility (Kartagener s syndrome). -

Brain Structural and Functional Asymmetry in Human Situs Inversus Totalis
Edinburgh Research Explorer Brain structural and functional asymmetry in human situs inversus totalis Citation for published version: Vingerhoets, G, Li, X, Hou, L, Bogaert, S, Verhelst, H, Gerrits, R, Siugzdaite, R & Roberts, N 2018, 'Brain structural and functional asymmetry in human situs inversus totalis' Brain Structure and Function. DOI: 10.1007/s00429-017-1598-5 Digital Object Identifier (DOI): 10.1007/s00429-017-1598-5 Link: Link to publication record in Edinburgh Research Explorer Document Version: Peer reviewed version Published In: Brain Structure and Function General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 24. Jul. 2019 Brain Structure and Function Brain Structural and Functional Asymmetry in Human Situs Inversus Totalis --Manuscript Draft-- Manuscript Number: BSAF-D-17-00474R1 Full Title: Brain Structural and Functional Asymmetry in Human Situs Inversus Totalis Article Type: Original Article Keywords: brain