United States Patent Office Patented Aug
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Report of the International Narcotics Control Board for 2010
Report of the International Narcotics Control Board involving treatment for cocaine abuse accounted for 510. According to the 2009 AIDS Epidemic Update, 65 per cent of all cases involving treatment for published by the Joint United Nations Programme on substance abuse in 1998, and that figure decreased, in HIV/AIDS and WHO, an estimated 29 per cent of the relative terms, to 49 per cent in 2008. For the past more than 2 million Latin Americans who abuse drugs 10 years, cocaine has been the primary drug of abuse by injection are infected with HIV. HIV epidemics among persons treated for drug problems in the region. among such drug abusers in the region tend to be concentrated in the Southern Cone. It is estimated that 506. Demand for “crack” cocaine appears to be in Argentina alone, almost half of the persons who emerging in some countries in South America. In 2008, abuse drugs by injection are infected with HIV. seizures of “crack” cocaine were reported in Argentina, Brazil, Chile, Paraguay and Venezuela (Bolivarian Republic of). In the Bolivarian Republic of Venezuela, C. Asia lifetime prevalence of the abuse of “crack” cocaine among the population aged 15-70 is 11.9 per cent. In East and South-East Asia that country, about a quarter of the persons who received treatment for drug addiction were addicted to 1. Major developments “crack” cocaine. In 2010, the Government of Brazil launched its integrated plan to combat “crack” cocaine 511. In East and South-East Asia, progress in reducing and other drugs. opium production is under threat, owing to an upswing in opium poppy cultivation during the 2009 growing 507. -

Research Brief New Reports, Bills and Updates of Latest Research
Research Brief New reports, bills and updates of latest research Parliamentary Library and Information Service Department of Parliamentary Services ISSN 1836-7828 (Print) 1836-8050 (Online) Number 3 February 2014 Drugs, Poisons and Controlled Substances (Poppy Cultivation and Processing) Amendment Bill 2013 This Research Brief includes the following sections: Introduction .............................................................................................................. 1 1. Second Reading Speech ...................................................................................... 1 2. Background ........................................................................................................... 2 History of the Opium Poppy and the Poppy Industry ........................................................... 2 Development of the Tasmanian Poppy Industry ..................................................................... 3 Regulation of the Poppy Industry and the Thebaine Poppy .................................................. 5 The Tasmanian Poppy Industry Today and Processor Plans for Expansion ...................... 7 Expanding the Poppy Industry to Victoria ................................................................................ 8 3. The Bill ................................................................................................................... 9 4. Other Jurisdiction ............................................................................................. 11 Appendix 1. Description of Morphine, -
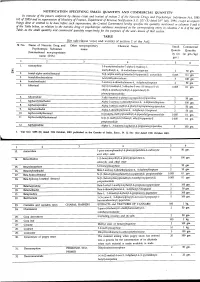
NOTIFICATION SPECIFYING SMALL QUANTITY and COMMERCIAL QUANTITYI Ly of the Pozoersconferred (Xxiiiil
NOTIFICATION SPECIFYING SMALL QUANTITY AND COMMERCIAL QUANTITYI ly of the pozoersconferred (xxiiiil . - lleyise by clansesfuiia) and of section2 of the Nnrcotic Drrtgsnnd pnlchotropic SrtbstancesAct, 19g5 (61 of 1985)and in supersessionof Ministry of Ftnnnce, (D Departmentof'Reuenue Noiification s.o.527 aotra i'6tt,1uli, tssi, exceptas respects things doneor omitted to be done .beforesuch supersession,the Cential Gooernmenihereby spectfiesthe qtnntitrl ,nlnt'ionedin coltnnnsS and 6 of the Tablebelow, in relationto.the narcoticdrug or psychotropicsubstnnce mentioned.ii tlr', ,1urrponding entnl in columns2 to 4 of thesaid Table,as the small quantitrl nnd commercialquantity respectiailyfor the purposesof the said clnuse:sof ttrit seciion. TABLE [Seesub-clause vii(a) and xxiii(a) of section 2 of the Act] Sl No. Name of Narcotic Drug and Other non-proprietary Chemical Name Small Commercial Psychotropic Substance name Quanti- Quantity (Intemational non-proprietary ity (in (in gm./kg.) name (INN) Acetorphine 3-0-acetyltetrahydro-7-alpha-(l-hydroxy-l- methylbutyt)-o, l4-endoetheno-onpavine $ 50 9.. 1\) Acetyl-alpha-methylfen N-[-(alpha-methylphenethyl)-4-piperidy]l acetanilide 0.1 g-. J. Acetyldihydrocodeine 100 4. Acetylmethadol 3-acetoxy-6-dimethylamino-4, 4 lheptane 50 gm. 5. Alfentanil -ethyl-4, N-[1-[2-( 5-dihydro-S-oxo-lH-tetrazol-t-yt) 01 gm. ethyll-4-(methoxymethyl)-4-piperidinyll -N- ylpropanamide Allyprodine 3-allyl Jmethyl-4-phenyl-4 Alpha-3-acetoxy-6-dimethylamino-4,Ld lheptane 100 gm Alpha-3-ethyl-l-methyl-4-phenyl-4-propionox 50 gm. 9.7' AlphamethadolArPnametnaool Alpha-6-dimethylamino-4, 4-diphenyl-3-heptanol 2 S0 gm. 10. Alphu-*"thylf"ntur,yl 11. -

Tennessee Drug Statutes Chart
Tennessee Drug Statutes Chart Tennessee Code: Title 39 Criminal Code SCHEDULE I OFFENSES/PENALTIES ENHANCEMENTS/ (*All sentences are for BENEFIT RESTRICTIONS standard offenders. Enhancement/mitigating factors may increase/reduce sentence. See sentencing statutes in appendix) 39-17-405 Criteria 39-17-417(b) (1) High potential for abuse; (2) Manufacture, delivery, No accepted med. Use in US or sale, possession w/ lacks accepted safety for med use intent (p.w.i.) of Schedule I Class B felony: 8-12yrs; <$100,000 39-17-406 Substances 3-17-417(i) Manufacture, (b) Opiates delivery, p.w.i. of heroin (c) Opium derivatives: E.g., (>15g); morphine heroin, codeine compounds, (>15g); hydromorphone morphine compounds, etc. (>5g); LSD (>5g); (d) Hallucinogenic substances: cocaine (>26g); E.g., MDMA, mescaline, DMT, pentazocine & peyote, LSD, psilocybin, synthetic tripelennamine (>5g); THC, etc. PCP (>30g); (e) Depressants: e.g., GHB, barbiturates (>100g); Qualuudes phenmetrazine (>50g); (f) Stimulants: E.g., fenethylline, amphetamine/ BZP methamphetamine (>26g); peyote (>1000g); Other Schedule I or II substances (>200g) Class B felony: 8-12yrs; <$200,000 SCHEDULE II 1 Tennessee Drug Statutes Chart Tennessee Code: Title 39 Criminal Code 39-17-407 Criteria 3-17-417(j) Manufacture, (1) high potential for abuse; (2) delivery, p.w.i. of heroin accepted med use in US w/ severe (>150g); morphine restrictions; and (3) abuse may (>150g); lead to severe psych or phys hydromorphone (>50g); dependence LSD (>50g); cocaine (>300g); pentazocine & tripelennamine (>50g); PCP (>300g); barbiturates (>1000g); phenmetrazine (>500g); amphetamine/ methamphetamine (>300g); peyote (>10000g); Other Schedule I or II substances (>2000g) Class A felony; 15-25yrs; <$500,000 39-17-408 Substances 39-17-417(c)(1) (b) Narcotics derived from Manufacture, delivery, vegetable origin or chemical p.w.i. -

Narcotic Drugs Stupefiants Estupefacientes
E/INCB/1993/21Supp.6 INTERNATIONAL NARCOTICS CONTROL BOARD - VIENNA SUPPLEMENT No. 6 TO NARCOTIC DRUGS ESTIMATED WORLD REQUIREMENTS FOR 1994 STATISTICS FOR 1992 ESTIMATES UPDATED AS OF 30 JUNE 1994 ORGANE INTERNATIONAL DE CONTROLE DES STUPEFIANTS - VIENNE SUPPLEMENT N° 6 A r STUPEFIANTS EVALUATIONS DES BESOINS DU MONDE POUR 1994 STATISTIQUES POUR 1992 EVALUATIONS A JOUR AU 30 JUlN 1994 JUNTA INTERNACIONAL DE FISCALlZACION DE ESTUPEFACIENTES - VIENA SUPLEMENTO N.o 6 A ESTUPEFACIENTES PREVISIONES DE LAS NECESIDADES MUNDIALES PARA 1994 ESTADfsTICAS PARA 1992 PREVISIONES ACTUALlZADAS AL 30 DE JUNIO DE 1994 ~If..~~ ~ ~-tR UNITED NATIONS - NATIONS UNIES - NACIONES UNIDAS 1994 The updating of Table A is carried out by means of 12 monthly supplements. In order to facilitate the task of the exporting countries, the 12 supplements now report all the totals of the estimates and not only the amended data. In this way, each supplement cancels and replaces the published table in its entirety. In order to accelerate the transmission of the supplements to the competent national authorities, the 12 supplements will appear in English. Reading of these 12 supplements in French and Spanish may be facilitated by consulting the indexes of countries and territories and of narcotic drugs appearing in the annual publication. La mise El jour du tableau A s'effectue au moyen de douze supplements mensuels. Afin de faciliter la tache des pays exportateurs, les douze supplements contiennent tous les totaux des evaluations et non pas seulement les chiffres qui ont ete modifies. De celte maniere, chaque supplement annule et remplace entierement le tableau publie. -

Legal Opium Production for Medical Use in Mexico: Options, Practicalities and Challenges
LEGAL OPIUM PRODUCTION FOR MEDICAL USE IN MEXICO: OPTIONS, PRACTICALITIES AND CHALLENGES. SUMMARY: • Many countries legally grow opium for the production of opioid medicines - the scale of this market historically matching illegal opium production for non-medical uses • Legal opium production, imports and exports take place under the auspices of the 1961 UN drug convention, overseen by the UN International Narcotics Control Board (INCB) • Turkey and India have successfully managed transitions of traditional small scale illegal opium producers into a legal production model for medical uses within the UN system • There are no practical reasons why Mexico could not produce opium for domestic markets (helping address domestic shortages), or for export • Mexico would face different circumstances and unique local challenges - and such a transition would need to be carefully managed as part of a wider social development program • Legal opium production in Mexico for medical uses would, however, not affect N. American demand for illegal non-medical uses, and it is likely that illegal opium production would simply be displaced • Part of a longer term solution could see interests North and South of the Mexico/US border coordinated - in line with the ‘shared responsibility’ philosophy - with legal Mexican opium production supplying innovative harm reduction responses to the opioid crisis in North America (including medical prescribing of opium, hydromorphone & heroin) 1. LEGAL OPIUM PRODUCTION: BACKGROUND. production of poppy straw (China producing raw opium and poppy straw), concentrate of poppy straw (CPS), and extracted alkaloids. Australia, Opium is the latex or gum extracted from the France, India, Spain and Turkey are the five main opium poppy (Papaver somniferum). -

Narcotic Drugs — Estimated World Requirements for 2018
English — Comments COMMENTS ON THE REPORTED STATISTICS ON NARCOTIC DRUGS Part two Part Summary The further overall reduction in global stocks and in the production of opium confirm the con- Deuxi tinuing trend towards the eventual elimination of the drug from the international market for è opiate raw material. me partie Poppy straw and concentrate of poppy straw derived from the two main varieties of poppy straw (the morphine-rich and thebaine-rich varieties) decreased slightly in 2016 compared Segunda parte with 2015, and the manufacture of morphine remained stable at 422.1 tons, of which around 87 per cent of global manufacture was converted into other narcotic drugs or into substances not covered by the 1961 Convention. Of the remaining 13 per cent, only 8.6 per cent was directly consumed for palliative purposes. The differences in consumption levels between countries continued to be very significant. In 2016, 80 per cent of the world population consumed only 14 per cent of the total amount of morphine used for the management of pain and suffering. Although that represented an improvement on 2014, when 80 per cent of the world population consumed 9.5 per cent, the disparity in consumption of narcotic drugs for palliative care continues to be a matter of concern. After some fluctuations in the preceding years, global manufacture of thebaine reached the record level of 156 tons in 2016, signalling that the demand for medicines derived from thebaine, after having decreased in the past several years, appears to have resumed, despite restrictions on prescription drugs recently imposed in the main market (the United States of America) in response to their abuse and the high number of overdose deaths they have caused. -

Opioid Analgesics PEDIATRIC PAIN MANAGEMENT
Opioid Analgesics PEDIATRIC PAIN MANAGEMENT Ardin S. Berger, D.O. Department of Anesthesiology & Pain Management Version 1, revised 10/12/19 Contents ▪ General characteristics ▪ Opioids in Renal Failure ▪ Common side effects ▪ Combination Medications ▪ Specific Medications › Acetaminophen Toxicity › Morphine ▪ Opioids & Substance Use Disorders › Fentanyl › Substance Use Disorder › Hydromorphone • Definitions › Sufentanil – Dependence › Oxycodone – Tolerance › Methadone › Heroin › Meperidine › Preventative Strategies › Tramadol › Nalbuphine › Naloxone Opioid Medications – Generalized Characteristics ▪ Opiates vs. Opioids › Opiates: substances with active ingredients naturally derived from opium • Morphine, codeine, thebaine › Opioids • Synthetically manufactured substances that mimic the effects of opium ▪ Classification based on action › Full agonists (primary action via μ1 receptors) › Partial agonists: less conformational change and receptor activation than full agonists • Low doses: may provide similar effects to full agonists • High doses: analgesic activity plateaus; increased adverse effects › Mixed agonists/antagonists: varying activity depending on opioid receptor and dose Mu Delta Kappa Clinical Effect Supraspinal chemical, Mechanical nociception Spinal-mediated thermal thermal, & mechanical Inflammatory pain nociception nociception Analgesia Chemical visceral pain Analgesia Euphoria Sedation Euphoria, sedation Physical dependence Miosis Respiratory Depression Dopamine release inhibition Dysphoria Miosis Mu receptor modulation -

United Nations Nations Vnies UNRESTRIC1'ed ECONOMIC CONS Ell E/CN .7/ 107 and ECONOMIQUE 6 April 19 1~8 SOCIAL COUNCIL ET SOCIAL ORIGINAL: ENGLISH
United Nations Nations Vnies UNRESTRIC1'ED ECONOMIC CONS ElL E/CN .7/ 107 AND ECONOMIQUE 6 April 19 1~8 SOCIAL COUNCIL ET SOCIAL ORIGINAL: ENGLISH COMMISSION ON NARC't 'I'IC DRCGS TIDRD SESSION LIMITATION OF THE PRODUC'I'ION OF RAW MATERIAlS (I tem 13 (a ) of the Provisional Agenda) ~ne Assist ant Secr et ary- r~nerel in charge of the t epart ment of Social Affairs has t he honour t o t r ansmit to t he membere of the Ccrrmi~sicn on Narcot i c Drugs t he f ollowing r epli es by Gove r~~ent s to t he ~us s t ionnai r8 on the Limit ation end Cont rol of the Cult i vation of t he Opium Poppy and Product i on of Raw Opi um, and t he Contr ol of ot ter Raw Mat er i al s us&d i n t he Manufactur e of Opium Alkaloi ds . /TABU'- OF CONTENTS E/CN.7//107 Page 2 TABLE OF CONTEIITS Page Explanatory note . 3 PART I - Opium-producing countries . 6 Gree_g_e 6 Indo- China 12 Japan. 17 Korea. 24 Turkey 32 YugQslavia 35 PART II - Countries utilizing poppy straw • 42 Australia. 42 France 45 The Netherlands. 49 Switzer land. 53 Czechoslovakia • 57 PART III - Ot her countries . 60 /COMMI SSION TI'./CN.7 /107 Po.ee 3 GOUHISSI ON ON II.ARCOTIC DRUGS THIIID SESSION LllflTATION OF THE PRODUCTION CIS' RAH MNl'ER IAIS REPLIES BY GOVERI'Ir-1EN'J.'S TO THE QUE.:."'l'I OHNAIRE ON T1IE LlMI TA'I'ION AND CON'..ITWL OF THE CULTIVATIOii OF THE OPIUM POPPY AIID PR<IOO'CTION OF RAW OPIUl-1 AliD THE CONTROL OF OTHl!!R Rli.W MATERIALS USED IN 'ffiE IJ.JIJID'l\CT'..mE OF OPIUM ALY..ALOIDS (DOCUNENTS E/251/Addo2, E/CN. -

Poppy Letter
April 3, 2019 Scott Gottlieb William P. Barr Commissioner Attorney General U.S. Food and Drug Administration U.S. Department of Justice 10903 New Hampshire Avenue 950 Pennsylvania Avenue, NW Silver Spring, MD 20993 Washington, DC 20530-0001 Frank Yiannas Uttam Dhillon Dep. Commissioner, Food Policy and Response Acting Administrator U.S. Food and Drug Administration U.S. Drug Enforcement Administration 10903 New Hampshire Avenue 800 K Street NW Suite 500 Silver Spring, MD 20993 Washington DC 20001 Susan Mayne Steven Tave Director Director Center for Food Safety and Applied Nutrition Office of Dietary Supplement Programs U.S. Food and Drug Administration U.S. Food and Drug Administration 5001 Campus Drive 5001 Campus Drive College Park MD 20740 College Park MD 20740 Dear Commissioner Gottlieb, Deputy Commissioner Yiannas, Director Mayne, Attorney General Barr, Acting Administrator Dhillon, and Director Tave: The Center for Science in the Public Interest (CSPI), a consumer group with more than 45 years of experience advocating for a healthier food system, urges you to take immediate action to stop the illegal importation and sale of contaminated poppy seeds (“unwashed” poppy seeds) and poppy seed pods. These dangerous products can contain high levels of morphine, codeine, thebaine, and other opiate alkaloids. The products pose grave health risks, particularly when the opiates are concentrated by brewing large amounts into a “tea.” The apparently increasing threat they pose has been overlooked for too long as a component of the ongoing opioid epidemic in that addicted patients have been known to use these products as substitutes for other opioids. Contaminated poppy seeds and poppy seed pods pose a serious and immediate hazard to consumers. -

Oripavine (Final Decision)
34th ECDD - 2006/6.3 oripavine (final decision) Oripavine (final decision) a. Introduction Oripavine, O3-demethylthebaine, is a phenanthrene alkaloid contained in various species of the genus Papaver, including P. bracteatum Lindl. and P. orientale L.. It is not produced by traditionally cultivated varieties of opium poppy (P. somniferum L.) and is therefore not found in opium. However, in the last decade, a variety (a strain) of P. somniferum was created by plant breeders with a high content of oripavine and is now cultivated commercially on a considerable scale. Oripavine was pre-reviewed by the 33rd ECDD in 2002. The reason for pre-review in 2002 was that oripavine is a substance that is convertible into thebaine, and because thebaine in its turn is convertible into morphine. Thebaine and morphine are both in Schedule I of the 1961 Convention, morphine because of its liability for abuse, and thebaine because it can be converted into substances that are morphine derivatives and are included in Schedule I of the 1961 Convention because of their liability for abuse. The Committee concluded from this that the conversion of oripavine into morphine derivatives was indirect and therefore it hesitated whether Article 3, para. 3 point iii of the 1961 Convention was applicable in this case. The Committee also remarked that the relation of this situation to the 1988 Convention is not clear, as the latter regulates substances frequently used in the production of narcotic drugs or psychotropic substances. Due to these uncertainties, the 33rd ECDD did not finalize this review, but included in its report the following proposal: "The Committee urged WHO to develop additional scheduling guidelines in consultation with appropriate bodies of the United Nations for clarifying issues related to the conversion of precursors into scheduled substances." However, supplementary Guidelines for the WHO review of dependence producing psychoactive substances for international control have not been established by WHO Executive Board, as will be discussed in a separate point on the agenda. -

The Growing Importance of Ukraine As a Transit Country for Heroin Trafficking
The Growing Importance of Ukraine As A Transit Country for Heroin Trafficking U.S.-Ukraine Research Cambridge, MA Lexington, MA Partnership Hadley, MA Bethesda, MD Washington, DC Chicago, IL Cairo, Egypt Johannesburg, South Africa Final Report June 2002 Prepared for National Institute of Justice International Center Abt Associates Inc. Prepared by 1110 Vermont Avenue, NW Mary Layne Suite 610 Mykola.S. Khruppa Washington, DC 20005-3522 Anatoly.A. Musyka Contents Acknowledgements...............................................................................................................................ii Preface ..................................................................................................................................................iii 1. Introduction................................................................................................................................1 2. Background ................................................................................................................................3 Scope of the Drug Problem in Ukraine........................................................................................3 Drug Abuse........................................................................................................................4 Anti-Drug Law Enforcement Mechanisms ..................................................................................7 3. Heroin Trafficking From Southwest Asia..............................................................................11