Receptor 6 Through Activation of the P2Y Expression in Microglia and Astrocytes -Diphosphate Induces Chemokine ′ Uridine 5
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Protective Effects of Anthocyanins on the Ectonucleotidase Activity in the Impairment of Memory Induced by Scopolamine in Adult Rats
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Life Sciences 91 (2012) 1221–1228 Contents lists available at SciVerse ScienceDirect Life Sciences journal homepage: www.elsevier.com/locate/lifescie Protective effects of anthocyanins on the ectonucleotidase activity in the impairment of memory induced by scopolamine in adult rats Jessié M. Gutierres a,⁎, Fabiano B. Carvalho a, Maria R.C. Schetinger a, Marília V. Rodrigues a, Roberta Schmatz a, Victor C. Pimentel a, Juliano M. Vieira a, Michele M. Rosa a, Patrícia Marisco a, Daniela A. Ribeiro a, Claudio Leal a, Maribel A. Rubin a, Cinthia M. Mazzanti c, Roselia Spanevello b,⁎⁎ a Departamento de Química, Universidade Federal de Santa Maria, Santa Maria, RS 97105-900, Brazil b Centro de Ciências Químicas, Farmacêuticas e de Alimentos, Universidade Federal de Pelotas, Campus Universitário-Capão do Leão 96010-900 Pelotas, RS, Brazil c Clínica de Pequenos animais, Centro de Ciências Rurais, Universidade Federal de Santa Maria, Santa Maria, RS 97105-900, Brazil article info abstract Article history: Aims: We investigated whether the treatment with anthocyanins prevents the scopolamine-induced memory Received 24 March 2012 deficits and whether ectonucleotidase activities and purine levels are altered in the cerebral cortex (CC) and Accepted 19 September 2012 hippocampus (HC) in this model of mnemonic deficit in rats. Main methods: The animals were divided into 4 experimental groups: control (vehicle), anthocyanins (Antho), Keywords: scopolamine (SCO), and scopolamine plus anthocyanins (SCO+Antho). After seven days of treatment, they Anthocyanins were tested in the inhibitory avoidance task and open field test and submitted to euthanasia. -

THE ROLE of CILOSTAZOL in ISCHEMIC STROKE
Since 1960 MedicineMedicine KoratKorat โรงพยาบาลมหาราชนครราชสีมา THE ROLE of CILOSTAZOL in ISCHEMIC STROKE PAWUT MEKAWICHAI MD DEPARTMENT of MEDICINE MAHARAT NAKHON RATCHASIMA HOSPITAL This presentation is supported by Thai Osuka CONTENT 1 Mechanism of cilostazol 2 Acute Ischemic Stroke (within 48h) 3 Secondary Prevention and Recurrence 4 Bleeding Complication CONTENT 1 Mechanism of cilostazol 2 Acute Ischemic Stroke (within 48h) 3 Secondary Prevention and Recurrence 4 Bleeding Complication Phosphodiesterase Inhibitor Increase cAMP → reduce platelet function Decrease cAMP → increase platelet function PDE enzyme change cAMP to 5-AMP Inhibit PDE enzyme → incrase cAMP → reduce platelet function Phosphodiesterase inhibitor Cilostazol Dipyridamole Classification of PDE Isozyme 9 family of PDE enzyme (PDE1-9) PDE-3 Site: platelet, heart, vascular SM, adipose tissue Inhibitor: Cilostazol PDE-5 Site: platelet, heart, vascular SM, corpus carvernosum Inhibitor: Dipyridamole, Sidenafil Distribution of PDE Isozyme Cell Platelet Heart Vascular Adipose SMC tissue Dominant III III III III Subordinate II V I II IV IV I II Inhibit PDE-3 antiplatelet action vasodilatation → headache ? inhibit vascular SMC proliferation tachycardia and palpitation increase HDL and decrease TG Which antiplatelet drug that can use in acute ischemic stroke? 1. ASA 81 mg 2. ASA 300 mg 3. Clopidogrel 4. Dipyridamole+ASA 5. Cilostazol CONTENT 1 Mechanism of cilostazol 2 Acute Ischemic Stroke (within 48h) 3 Secondary Prevention and Recurrence 4 Bleeding Complication ASPIRIN International Stroke Trial (IST) 1 ASA 300 mg-reduce mortality and recurrence in 2 wk) Chinese Acute Stroke Trial (CAST) 2 ASA 160 mg-reduce mortality and recurrence) reduce mortality rate 9/1,000 OR 0.92 (0.87-0.98) good functional outcome 7/1,000 OR 1.02 (1.01-1.04) increase severe bleeding (within 2-4 wk) 4/1,000 OR 1.69 (1.35-2.11) no evidence for difference dose of ASA 1.Lancet 1997; 349: 1569-81. -

Ectonucleotidase Expression on Human Amnion Epithelial Cells
Ectonucleotidase Expression on Human Amnion Epithelial Cells: Adenosinergic Pathways and Dichotomic Effects on Immune Effector Cell Populations This information is current as of September 28, 2021. Fabio Morandi, Alberto L. Horenstein, Valeria Quarona, Angelo Corso Faini, Barbara Castella, Raghuraman C. Srinivasan, Stephen C. Strom, Fabio Malavasi and Roberto Gramignoli J Immunol published online 26 December 2018 Downloaded from http://www.jimmunol.org/content/early/2018/12/23/jimmun ol.1800432 http://www.jimmunol.org/ Supplementary http://www.jimmunol.org/content/suppl/2018/12/23/jimmunol.180043 Material 2.DCSupplemental Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on September 28, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2018 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Published December 26, 2018, doi:10.4049/jimmunol.1800432 The Journal of Immunology Ectonucleotidase Expression on Human Amnion Epithelial Cells: Adenosinergic Pathways and Dichotomic Effects on Immune Effector Cell Populations Fabio Morandi,*,1 Alberto L. Horenstein,†,‡,x,1 Valeria Quarona,† Angelo Corso Faini,† Barbara Castella,† Raghuraman C. -

Characterization of the Contractile P2Y14 Receptor in Mouse Coronary and Cerebral Arteries ⇑ Kristian Agmund Haanes , Lars Edvinsson
FEBS Letters 588 (2014) 2936–2943 journal homepage: www.FEBSLetters.org Characterization of the contractile P2Y14 receptor in mouse coronary and cerebral arteries ⇑ Kristian Agmund Haanes , Lars Edvinsson Department of Clinical Experimental Research, Glostrup Research Institute, Copenhagen University Hospital, Nordre Ringvej 59, 2600 Glostrup, Denmark article info abstract Article history: Extracellular UDP-glucose can activate the purinergic P2Y14 receptor. The aim of the present study Received 13 February 2014 was to examine the physiological importance of P2Y14 receptors in the vasculature. The data pre- Revised 13 May 2014 sented herein show that UDP-glucose causes contraction in mouse coronary and basilar arteries. Accepted 21 May 2014 The EC values and immunohistochemistry illustrated the strongest P2Y14 receptor expression in Available online 6 June 2014 50 the basilar artery. In the presence of pertussis toxin, UDP-glucose inhibited contraction in coronary Edited by Ned Mantei arteries and in the basilar artery it surprisingly caused relaxation. After organ culture of the coro- nary artery, the EC50 value decreased and an increased staining for the P2Y14 receptor was observed, showing receptor plasticity. Keywords: Purinergic receptors Ó 2014 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved. P2Y14 Coronary artery Basilar artery Receptor upregulation P2Y2 KO 1. Introduction vasoconstriction in porcine pancreatic arteries [12]. This study was designed to examine the physiological importance -

In Vitro Modulation of Cisplatin Accumulation in Human Ovarian Carcinoma Cells by Pharmacologic Alteration of Microtubules
In vitro modulation of cisplatin accumulation in human ovarian carcinoma cells by pharmacologic alteration of microtubules. R D Christen, … , D R Shalinsky, S B Howell J Clin Invest. 1993;92(1):431-440. https://doi.org/10.1172/JCI116585. Research Article We have previously shown that forskolin and 3-isobutyl-1-methylxanthine (IBMX) increased accumulation of cisplatin (DDP) in DDP-sensitive 2008 human ovarian carcinoma cells in proportion to their ability to increase cAMP. Since the major function of cAMP is to activate protein kinase A, it was conjectured that the stimulation of DDP accumulation was mediated by a protein kinase A substrate. We now show that exposure of 2008 cells to forskolin resulted in phosphorylation of a prominent 52-kD membrane protein. Microsequencing of the band demonstrated it to be human beta-tubulin. Similarly, pretreatment of 2008 cells with the microtubule stabilizing drug taxol increased platinum accumulation in a dose-dependent manner. In 11-fold DDP-resistant 2008/C13*5.25 cells, decreased DDP accumulation was associated with enhanced spontaneous formation of microtubule bundles and decreased expression of beta-tubulin and the tubulin-associated p53 antioncogene relative to 2008 cells. 2008/C13*5.25 cells had altered sensitivity to tubulin- binding drugs, being hypersensitive to taxol and cross-resistant to colchicine. We conclude that pharmacologic alterations of tubulin enhance accumulation of DDP, and that the DDP-resistant phenotype in 2008/C13*5.25 cells is associated with tubulin abnormalities. Find the latest version: https://jci.me/116585/pdf In Vitro Modulation of Cisplatin Accumulation in Human Ovarian Carcinoma Cells by Pharmacologic Alteration of Microtubules Randolph D. -

MEDICINE to TREAT: HEART DISEASES Antiplatelet Agents Aspirin Dipyridamole Clopidogrel Ticlopidine 1. What Are These Medicines U
PATIENT INFORMATION LEAFLET MEDICINE TO TREAT: HEART DISEASES Antiplatelet Agents Aspirin Dipyridamole Clopidogrel Ticlopidine 1. What are these medicines used for? Medicine Purpose of medicine Brand Names This medicine makes blood less sticky to reduce the chance of blood forming Aspirin Cardiprin® clots which will lead to stroke or heart Disprin® attack. This medicine makes the blood less Dipyridamole sticky to reduce the chance of stroke. It Persantin® is used together with aspirin. These medicines make your blood less Clopidogrel (Plavix®) Clopidogrel sticky to reduce chance of stroke or Ticlopidine (Ticlid®), Ticlopidine heart attack. They can be used alone or together with aspirin. 2. How should I take the medicines? • Do not stop taking your medicines without checking with your doctors. • If you miss a dose, take the missed dose as soon as you remember. If it is almost time for your next dose, take only the usual dose. Do not double your dose or use extra medicine to make up for the missed dose. • You should take your medicine after a meal to prevent stomach upset. • How you take aspirin depends on the brand and type of aspirin the doctor gives you: o For tablets which can be chewed: Take with food or glass of water or milk These tablets may be chewed or swallowed whole The solution may also be used as a gargle. Please check with your pharmacist on how to use it as a gargle. o For tablets which enteric coated: Enteric coated forms of aspirin reduce stomach upset that is caused by the medicine Take with a glass of water after food. -

Pyridylisatogen Tosylate of ATP-Responses at a Recombinant P2y1 Purinoceptor 1B.F
British Journal of Pharmacology (I996) 117, 1111 1118 1996 Stockton Press All rights reserved 0007-1188/96 $12.00 0 Potentiation by 2,2'-pyridylisatogen tosylate of ATP-responses at a recombinant P2y1 purinoceptor 1B.F. King, *C. Dacquet, A.U. Ziganshin, tD.F. Weetman, G. Burnstock, *P.M. Vanhoutte & *M. Spedding Department ofAnatomy and Developmental Biology, University College London, Gower Street, London WClE 6BT; *Institute de Recherches Servier, 125 Chemin de Ronde, Croissy sur Seine, 72890, France and tDepartment of Pharmacology, University of Sunderland, Tyne and Wear SRI 3SD 1 2,2'-Pyridylisatogen tosylate (PIT) has been reported to be an irreversible antagonist of responses to adenosine 5'-triphosphate (ATP) at metabotropic purinoceptors (of the P2Y family) in some smooth muscles. When a recombinant P2Y purinoceptor (derived from chick brain) is expressed in Xenopus oocytes, ATP and 2-methylthioATP (2-MeSATP) evoke calcium-activated chloride currents (IC1,ca) in a concentration-dependent manner. The effects of PIT on these agonist responses were examined at this cloned P2Y purinoceptor. 2 PIT (0.1-100 rM) failed to stimulate P2y, purinoceptors directly but, over a narrow concentration range (0.1-3 tM), caused a time-dependent potentiation (2-5 fold) of responses to ATP. The potentiation of ATP-responses by PIT was not caused by inhibition of oocyte ecto-ATPase. At high concentrations (3-100 gM), PIT irreversibly inhibited responses to ATP with a IC_0 value of 13 +9 gM (pKB= 4.88 + 0.22; n = 3). PIT failed to potentiate inward currents evoked by 2-MeSATP and only inhibited the responses to this agonist in an irreversible manner. -

Clinical Update
Atualização Clínica Antiagregantes Plaquetários na Prevenção Primária e Secundária de Eventos Aterotrombóticos Platelet Antiaggregants in Primary and Secondary Prevention of Atherothrombotic Events Marcos Vinícius Ferreira Silva1, Luci Maria SantAna Dusse1, Lauro Mello Vieira, Maria das Graças Carvalho1 Departamento de Análises Clínicas e Toxicológicas, Faculdade de Farmácia, Universidade Federal de Minas Gerais1, Belo Horizonte, MG – Brasil Resumo prevenção primária e secundária de tais eventos. Os fatores A aterotrombose e suas complicações correspondem, de risco associados ao desenvolvimento desses eventos hoje, à principal causa de mortalidade no mundo todo, estão intimamente associados à exacerbação da ativação e sua incidência encontra-se em franca expansão. plaquetária que, por sua vez, favorece a formação de As plaquetas desempenham um papel essencial na agregados plaquetários e geração de trombina, resultando patogênese dos eventos aterotrombóticos, justificando a em trombos ricos em plaquetas (trombos brancos). Dessa utilização dos antiagregantes plaquetários na prevenção forma, o uso de antiagregantes plaquetários tem sido dos mesmos. Desse modo, é essencial que se conheça o benéfico na prevenção primária e secundária de eventos perfil de eficácia e segurança desses fármacos em prevenção mediados por trombos. primária e secundária de eventos aterotrombóticos. Dentro As características dos principais antiagregantes plaquetários desse contexto, a presente revisão foi realizada com o utilizados na prática clínica e em fase de estudos estão objetivo de descrever e sintetizar os resultados dos principais descritos na Tabela 13-9, e as proteínas de membrana com as ensaios, envolvendo a utilização de antiagregantes nos dois quais eles interagem e as vias metabólicas nas quais atuam níveis de prevenção, e avaliando a eficácia e os principais são ilustrados Figura 110. -

Ectonucleotidase Activities in Sertoli Cells from Immature Rats
Brazilian Journal of Medical and Biological Research (2001) 34: 1247-1256 Ectonucleotidases in Sertoli cells 1247 ISSN 0100-879X Ectonucleotidase activities in Sertoli cells from immature rats E.A. Casali, T.R. da Silva, Departamento de Bioquímica, Instituto de Ciências Básicas da Saúde, D.P. Gelain, G.R.R.F. Kaiser, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brasil A.M.O. Battastini, J.J.F. Sarkis and E.A. Bernard Abstract Correspondence Sertoli cells have been shown to be targets for extracellular purines Key words E.A. Bernard such as ATP and adenosine. These purines evoke responses in Sertoli · Sertoli cells Departamento de Bioquímica · Purine nucleotides cells through two subtypes of purinoreceptors, P2Y2 and PA1. The Rua Ramiro Barcellos, 2600, Anexo · signals to purinoreceptors are usually terminated by the action of ATP diphosphohydrolase 90035-003 Porto Alegre, RS · Ecto-5’-nucleotidase ectonucleotidases. To demonstrate these enzymatic activities, we Brasil · Ectoadenosine deaminase Fax: +55-51-316-5540 cultured rat Sertoli cells for four days and then used them for different E-mail: [email protected] assays. ATP, ADP and AMP hydrolysis was estimated by measuring the Pi released using a colorimetric method. Adenosine deaminase activity (EC 3.5.4.4) was determined by HPLC. The cells were not disrupted after 40 min of incubation and the enzymatic activities were Received September 6, 2000 considered to be ectocellularly localized. ATP and ADP hydrolysis Accepted July 2, 2001 was markedly increased by the addition of divalent cations to the reaction medium. A competition plot demonstrated that only one enzymatic site is responsible for the hydrolysis of ATP and ADP. -
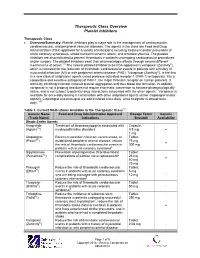
Therapeutic Class Overview Platelet Inhibitors
Therapeutic Class Overview Platelet Inhibitors Therapeutic Class • Overview/Summary: Platelet inhibitors play a major role in the management of cardiovascular, cerebrovascular, and peripheral vascular diseases. The agents in the class are Food and Drug Administration (FDA)-approved for a variety of indications including treatment and/or prevention of acute coronary syndromes, stroke/transient ischemic attack, and thrombocythemia. The platelet inhibitors are also indicated to prevent thrombosis in patients undergoing cardiovascular procedures and/or surgery. The platelet inhibitors exert their pharmacologic effects through several different mechanisms of action.1-8 The newest platelet inhibitor to be FDA-approved is vorapaxar (Zontivity®), which is indicated for the reduction of thrombotic cardiovascular events in patients with a history of myocardial infarction (MI) or with peripheral arterial disease (PAD).7 Vorapaxar (Zontivity®), is the first in a new class of antiplatelet agents called protease-activated receptor-1 (PAR-1) antagonists. It is a competitive and selective antagonist of PAR-1, the major thrombin receptor on human platelets. It works by inhibiting thrombin-induced platelet aggregation and thus blood clot formation. In addition, vorapaxar is not a prodrug and does not require enzymatic conversion to become pharmacologically active, and is not subject to potential drug interactions associated with the other agents.7 Vorapaxar is available for once-daily dosing in combination with other antiplatelet agents (either clopidogrel -

P2X7 in Cancer: from Molecular Mechanisms to Therapeutics
REVIEW published: 04 June 2020 doi: 10.3389/fphar.2020.00793 P2X7 in Cancer: From Molecular Mechanisms to Therapeutics Romain Lara 1*, Elena Adinolfi 2, Catherine A. Harwood 3, Mike Philpott 4, Julian A. Barden 5, Francesco Di Virgilio 6 and Shaun McNulty 1 1 Biosceptre (UK) Limited, Cambridge, United Kingdom, 2 Department of Medical Sciences, University of Ferrara, Ferrara, Italy, 3 Centre for Cell Biology and Cutaneous Research, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London, United Kingdom, 4 Centre for Cutaneous Research, Blizard Institute, Bart’s & The London School of Medicine and Dentistry, Queen Mary University of London, London, United Kingdom, 5 Biosceptre (Australia) Pty Ltd., Sydney, NSW, Australia, 6 Department of Morphology, Surgery and Experimental Medicine, Section of Pathology, Oncology and Experimental Biology, University of Ferrara, Ferrara, Italy P2X7 is a transmembrane receptor expressed in multiple cell types including neurons, dendritic cells, macrophages, monocytes, B and T cells where it can drive a wide range of physiological responses from pain transduction to immune response. Upon activation by Edited by: its main ligand, extracellular ATP, P2X7 can form a nonselective channel for cations to Stefan Feske, enter the cell. Prolonged activation of P2X7, via high levels of extracellular ATP over an New York University, United States extended time period can lead to the formation of a macropore, leading to depolarization Reviewed by: Friedrich Haag, of the plasma membrane and ultimately to cell death. Thus, dependent on its activation University Medical Center Hamburg- state, P2X7 can either drive cell survival and proliferation, or induce cell death. -

Preventive Drug List
PREVENTIVE DRUG LIST Preventive medications are used for the prevention of conditions such as high blood pressure, high cholesterol, diabetes, asthma, osteoporosis, heart attack, stroke and prenatal nutrient deficiency. Following is a list of generic preventive medications covered at 100%, arranged by type of condition. diltiazem sotalol AF glipizide Asthma related diltiazem ER sotalol HCl glipizide ER albuterol sulfate doxazosin mesylate spironolactone glipizide/metformin HCl albuterol sulfate (nebulizer enalapril maleate spironolactone/hctz glyburide solution) enalapril maleate/hctz telmisartan glyburide micronized albuterol sulfate/ipratropium eplerenone telmisartan/amlodipine glyburide/metformin nebulizer solution eprosartan mesylate telmisartan/hctz Humalog budesonide felodipine ER terazosin HCl Humulin caffeine citrate fosinopril sodium timolol maleate Invokamet cromolyn sodium inhalation fosinopril sodium/hctz torsemide Invokamet XR solution furosemide trandolapril Invokana ipratropium bromide guanfacine HCl trandolapril/verapamil Janumet levalbuterol HCl hydralazine HCl triamterene/hctz Janumet XR levalbuterol tartrate HFA hydrochlorothiazide valsartan Januvia metaproterenol sulfate indapamide valsartan/hctz Kombiglyze XR montelukast irbesartan Vecamyl – mecamylamine HCl Lantus terbutaline sulfate irbesartan/hctz Verapamil Lantus SoloStar TheoChron isradipine Levemir theophylline anhydrous labetalol HCl Blood thinner related Levemir FlexTouch zafirlukast lisinopril aspirin/dipyridamole ER metformin HCl lisinopril/hctz cilostazol