Reconstructing Equid Mobility in Miocene Florida Using Strontium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Three-Toed Browsing Horse Anchitherium (Equidae) from the Miocene of Panama
J. Paleonl., 83(3), 2009, pp. 489-492 Copyright © 2009, The Paleontological Society 0022-3360/09/0083-489S03.00 THREE-TOED BROWSING HORSE ANCHITHERIUM (EQUIDAE) FROM THE MIOCENE OF PANAMA BRUCE J. MACFADDEN Florida Museum of Natural History, University of Florida, Gainesville FL 32611, <[email protected]> INTRODUCTION (CRNHT/APL); L, left; M, upper molar; R upper premolar; R, DURING THE Cenozoic, the New World tropics supported a rich right; TRN, greatest transverse width. biodiversity of mammals. However, because of the dense SYSTEMATIC PALEONTOLOGY vegetative ground cover, today relatively little is known about extinct mammals from this region (MacFadden, 2006a). In an Class MAMMALIA Linnaeus, 1758 exception to this generalization, fossil vertebrates have been col- Order PERISSODACTYLA Owen, 1848 lected since the second half of the twentieth century from Neo- Family EQUIDAE Gray, 1821 gene exposures along the Panama Canal. Whitmore and Stewart Genus ANCHITHERIUM Meyer, 1844 (1965) briefly reported on the extinct land mammals collected ANCHITHERIUM CLARENCI Simpson, 1932 from the Miocene Cucaracha Formation that crops out in the Gail- Figures 1, 2, Table 1 lard Cut along the southern reaches of the Canal. MacFadden Referred specimen.—UF 236937, partial palate (maxilla) with (2006b) formally described this assemblage, referred to as the L P1-M3, R P1-P3, and small fragment of anterointernal part of Gaillard Cut Local Fauna (L.E, e.g., Tedford et al., 2004), which P4 (Fig. 1). Collected by Aldo Rincon of the Smithsonian Tropical consists of at least 10 species of carnivores, artiodactyls (also see Research Institute, Republic of Panama, on 15 May 2008. -

Exhibit Specimen List FLORIDA SUBMERGED the Cretaceous, Paleocene, and Eocene (145 to 34 Million Years Ago) PARADISE ISLAND
Exhibit Specimen List FLORIDA SUBMERGED The Cretaceous, Paleocene, and Eocene (145 to 34 million years ago) FLORIDA FORMATIONS Avon Park Formation, Dolostone from Eocene time; Citrus County, Florida; with echinoid sand dollar fossil (Periarchus lyelli); specimen from Florida Geological Survey Avon Park Formation, Limestone from Eocene time; Citrus County, Florida; with organic layers containing seagrass remains from formation in shallow marine environment; specimen from Florida Geological Survey Ocala Limestone (Upper), Limestone from Eocene time; Jackson County, Florida; with foraminifera; specimen from Florida Geological Survey Ocala Limestone (Lower), Limestone from Eocene time; Citrus County, Florida; specimens from Tanner Collection OTHER Anhydrite, Evaporite from early Cenozoic time; Unknown location, Florida; from subsurface core, showing evaporite sequence, older than Avon Park Formation; specimen from Florida Geological Survey FOSSILS Tethyan Gastropod Fossil, (Velates floridanus); In Ocala Limestone from Eocene time; Barge Canal spoil island, Levy County, Florida; specimen from Tanner Collection Echinoid Sea Biscuit Fossils, (Eupatagus antillarum); In Ocala Limestone from Eocene time; Barge Canal spoil island, Levy County, Florida; specimens from Tanner Collection Echinoid Sea Biscuit Fossils, (Eupatagus antillarum); In Ocala Limestone from Eocene time; Mouth of Withlacoochee River, Levy County, Florida; specimens from John Sacha Collection PARADISE ISLAND The Oligocene (34 to 23 million years ago) FLORIDA FORMATIONS Suwannee -

Annualreportofdi72fiel.Pdf
mmmmm , THE UNIVERSITY OF ILLINOIS LIBRARY SOT CENTRAL CIRCULATION BOOKSTACKS The person charging this material is re- sponsible for its renewal or its return to the library from which it was borrowed on or before the Latest Date stamped below. The Minimum Fee for each Lost Book is $50.00. Theft/ mutilation, and underlining of books are reasons for disciplinary action and may result in dismissal from the University. TO RENEW CALL TELEPHONE CENTER, 333-8400 UNIVERSITY OF ILLINOIS LIBRARY AT URBANA-CHAMPAIGN MAR 9 1991 When renewing by phone, write new due date below previous due date. L162 Field Museum of Natural History Reports, Vol. VII, Plate XXI ERNEST R. GRAHAM Trustee of the Museum and member of the Building Committee Field Museum of Natural History Founded by Marshall Field, 1893 Publication 248 Report Series Vol. VII, No. 2 ANNUAL REPORT OF THE DIRECTOR TO THE BOARD OF TRUSTEES FOR THE YEAR 1927 nf -"^ THE imm JUL 3 1323 Of 'LUNOIS UNlVWSltY Chicago, U. S. A. January, 1928 OF THE tiW^ViiuSHY Of ILimOlS PRINTED IN THE UNITED STATES OF AMERICA BY FIELD MUSEUM PRESS ^ i V BEQUESTS Bequests to Field Museum of Natural History may be made in securities, money, books or collections. They may, if desired, take the form of a memorial to the memory of a person or cause, to be named by the giver. For those desirous of making bequests to the Museum, the following form is suggested: FORM OF BEQUEST I do hereby give and bequeath to Field Museum of Natural History of the City of Chicago, State of Illinois, Cash contributions made within the taxable year to Field Museum of Natural History to an amount not in excess of 15 per cent of the taxpayer's net income are allowable as deduc- tions in computing net income under Article 251 of Regula- tion 69 relating to the income tax under the Revenue Act of 1926. -

Paleobiology of Archaeohippus (Mammalia; Equidae), a Three-Toed Horse from the Oligocene-Miocene of North America
PALEOBIOLOGY OF ARCHAEOHIPPUS (MAMMALIA; EQUIDAE), A THREE-TOED HORSE FROM THE OLIGOCENE-MIOCENE OF NORTH AMERICA JAY ALFRED O’SULLIVAN A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2002 Copyright 2002 by Jay Alfred O’Sullivan This study is dedicated to my wife, Kym. She provided all of the love, strength, patience, and encouragement I needed to get this started and to see it through to completion. She also provided me with the incentive to make this investment of time and energy in the pursuit of my dream to become a scientist and teacher. That incentive comes with a variety of names - Sylvan, Joanna, Quinn. This effort is dedicated to them also. Additionally, I would like to recognize the people who planted the first seeds of a dream that has come to fruition - my parents, Joseph and Joan. Support (emotional, and financial!) came to my rescue also from my other parents—Dot O’Sullivan, Jim Jaffe and Leslie Sewell, Bill and Lois Grigsby, and Jerry Sewell. To all of these people, this work is dedicated, with love. ACKNOWLEDGMENTS I thank Dr. Bruce J. MacFadden for suggesting that I take a look at an interesting little fossil horse, for always having fresh ideas when mine were dry, and for keeping me moving ever forward. I thank also Drs. S. David Webb and Riehard C. Hulbert Jr. for completing the Triple Threat of Florida Museum vertebrate paleontology. In each his own way, these three men are an inspiration for their professionalism and their scholarly devotion to Florida paleontology. -

Soil Survey of Pinellas County, Florida
United States In cooperation with Department of the University of Florida, Agriculture Institute of Food and Soil Survey of Agricultural Sciences, Natural Agricultural Experiment Pinellas County, Resources Stations, and Soil and Conservation Water Science Service Department; the Florida Florida Department of Agricultural and Consumer Services; and the Pinellas County Board of Commissioners i How To Use This Soil Survey Detailed Soil Maps The detailed soil maps can be useful in planning the use and management of small areas. To find information about your area of interest, locate that area on the Index to Map Sheets. Note the number of the map sheet and turn to that sheet. Locate your area of interest on the map sheet. Note the map unit symbols that are in that area. Turn to the Contents, which lists the map units by symbol and name and shows the page where each map unit is described. The Contents shows which table has data on a specific land use for each detailed soil map unit. Also see the Contents for sections of this publication that may address your specific needs. ii This soil survey is a publication of the National Cooperative Soil Survey, a joint effort of the United States Department of Agriculture and other Federal agencies, State agencies including the Agricultural Experiment Stations, and local agencies. The Natural Resources Conservation Service (formerly the Soil Conservation Service) has leadership for the Federal part of the National Cooperative Soil Survey. Major fieldwork for this soil survey was completed in 2002. Soil names and descriptions were approved in 2003. Unless otherwise indicated, statements in this publication refer to conditions in the survey area in 2003. -

La Brea and Beyond: the Paleontology of Asphalt-Preserved Biotas
La Brea and Beyond: The Paleontology of Asphalt-Preserved Biotas Edited by John M. Harris Natural History Museum of Los Angeles County Science Series 42 September 15, 2015 Cover Illustration: Pit 91 in 1915 An asphaltic bone mass in Pit 91 was discovered and exposed by the Los Angeles County Museum of History, Science and Art in the summer of 1915. The Los Angeles County Museum of Natural History resumed excavation at this site in 1969. Retrieval of the “microfossils” from the asphaltic matrix has yielded a wealth of insect, mollusk, and plant remains, more than doubling the number of species recovered by earlier excavations. Today, the current excavation site is 900 square feet in extent, yielding fossils that range in age from about 15,000 to about 42,000 radiocarbon years. Natural History Museum of Los Angeles County Archives, RLB 347. LA BREA AND BEYOND: THE PALEONTOLOGY OF ASPHALT-PRESERVED BIOTAS Edited By John M. Harris NO. 42 SCIENCE SERIES NATURAL HISTORY MUSEUM OF LOS ANGELES COUNTY SCIENTIFIC PUBLICATIONS COMMITTEE Luis M. Chiappe, Vice President for Research and Collections John M. Harris, Committee Chairman Joel W. Martin Gregory Pauly Christine Thacker Xiaoming Wang K. Victoria Brown, Managing Editor Go Online to www.nhm.org/scholarlypublications for open access to volumes of Science Series and Contributions in Science. Natural History Museum of Los Angeles County Los Angeles, California 90007 ISSN 1-891276-27-1 Published on September 15, 2015 Printed at Allen Press, Inc., Lawrence, Kansas PREFACE Rancho La Brea was a Mexican land grant Basin during the Late Pleistocene—sagebrush located to the west of El Pueblo de Nuestra scrub dotted with groves of oak and juniper with Sen˜ora la Reina de los A´ ngeles del Rı´ode riparian woodland along the major stream courses Porciu´ncula, now better known as downtown and with chaparral vegetation on the surrounding Los Angeles. -

Florida Fossil Horse Newsletter
Florida Fossil horse Newsletter Volume 10, Number 1, 1st Half 2001 What's Inside? Fossil Horses On the Road: Archaeohippus and Parahippus Check Out the Bluegrass State Meet Our Artists Volunteers Help the Museum While Enhancing Their Own Education Thunderbeasts, Sexual Selection and Extinction Skeleton "Under Construction" Homeschooling Groups Request More Family Days at Thomas Farm Book Review - The Fossil Vertebrates of Florida Introducing A New Logo For Pony Express's 10th Anniversary Fossil Horses On the Road: Archaeohippus and Parahippus Check Out the Bluegrass State Lexington, Kentucky welcomed Miocene horses at a special event at the Lexington Children's Museum, March 3, 2001. Loaned by the Florida Museum of Natural History, many fossils of the two small prehistoric horses from Thomas Farm are being displayed at the Children's Museum through March and April. Touchable casts of the skulls and feet are also charming the kids, who think the "little horses" are just awesome. At the all-day fossil event, a large display was set up with a case for some of the more fragile horse Seth Woodring, 3, of Winchester, made himself a plaster "fossil" with some help from his mother, fossils. Three Beth, and 5-year-old sister, Rayne. David Stephenson photo (reprinted with permission from tables held fossil Herald Leader) bones and casts, with modern horse bones for comparison. Experts Dr. Teri Lear and Dr. Lenn Harrison, with the Department of Veterinary Science at the University of Kentucky, presented Archaeohippus and Parahippus to the public. Teri has participated in several digs at Thomas Farm, and talked with visitors about the Miocene digs and fossils. -
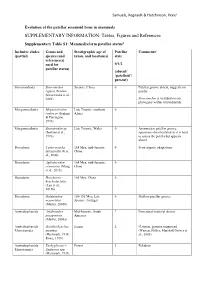
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels, Regnault & Hutchinson, PeerJ Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammaliaform patellar status$ Inclusive clades Genus and Stratigraphic age of Patellar Comments# (partial) species (and taxon, and location(s) state reference(s) used for 0/1/2 patellar status) (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic, China 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska et al., 2004) Sinoconodon is included on our phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji et China al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius (Meng China et al., 2015) Docodonta Docofossor 160 Mya, China 0 brachydactylus (Luo et al., 2015b) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Australosphenida Tachyglossus -

A New Machairodont from the Palmetto Fauna (Early Pliocene) of Florida, with Comments on the Origin of the Smilodontini (Mammalia, Carnivora, Felidae)
A New Machairodont from the Palmetto Fauna (Early Pliocene) of Florida, with Comments on the Origin of the Smilodontini (Mammalia, Carnivora, Felidae) Steven C. Wallace1*, Richard C. Hulbert Jr.2 1 Department of Geosciences, Don Sundquist Center of Excellence in Paleontology, East Tennessee State University, Johnson City, Tennessee, United States of America, 2 Florida Museum of Natural History, University of Florida, Gainesville, Florida, United States of America Abstract South-central Florida’s latest Hemphillian Palmetto Fauna includes two machairodontine felids, the lion-sized Machairodus coloradensis and a smaller, jaguar-sized species, initially referred to Megantereon hesperus based on a single, relatively incomplete mandible. This made the latter the oldest record of Megantereon, suggesting a New World origin of the genus. Subsequent workers variously accepted or rejected this identification and biogeographic scenario. Fortunately, new material, which preserves previously unknown characters, is now known for the smaller taxon. The most parsimonious results of a phylogenetic analysis using 37 cranio-mandibular characters from 13 taxa place it in the Smilodontini, like the original study; however, as the sister-taxon to Megantereon and Smilodon. Accordingly, we formally describe Rhizosmilodon fiteae gen. et sp. nov. Rhizosmilodon, Megantereon, and Smilodon ( = Smilodontini) share synapomorphies relative to their sister-taxon Machairodontini: serrations smaller and restricted to canines; offset of P3 with P4 and p4 with m1; complete verticalization of mandibular symphysis; m1 shortened and robust with widest point anterior to notch; and extreme posterior ‘‘lean’’ to p3/p4. Rhizosmilodon has small anterior and posterior accessory cusps on p4, a relatively large lower canine, and small, non-procumbent lower incisors; all more primitive states than in Megantereon and Smilodon. -

Late Miocene Tapirus(Mammalia
Bull. Fla. Mus. Nat. Hist. (2005) 45(4): 465-494 465 LATE MIOCENE TAPIRUS (MAMMALIA, PERISSODACTYLA) FROM FLORIDA, WITH DESCRIPTION OF A NEW SPECIES, TAPIRUS WEBBI Richard C. Hulbert Jr.1 Tapirus webbi n. sp. is a relatively large tapir from north-central Florida with a chronologic range of very late Clarendonian (Cl3) to very early Hemphillian (Hh1), or ca. 9.5 to 7.5 Ma. It is about the size of extant Tapirus indicus but with longer limbs. Tapirus webbi differs from Tapirus johnsoni (Cl3 of Nebraska) by its larger size, relatively shorter diastema, thicker nasal, and better developed transverse lophs on premolars. Tapirus webbi is more similar to Tapirus simpsoni from the late early Hemphillian (Hh2, ca. 7 Ma) of Nebraska, but differs in having narrower upper premolars and weaker transverse lophs on P1 and P2. Tapirus webbi differs from North American Plio-Pleistocene species such as Tapirus veroensis and Tapirus haysii in its polygonal (not triangu- lar) interparietal, spicular posterior lacrimal process, relatively narrow P2-M3, and lack of an extensive meatal diverticulum fossa on the dorsal surface of the nasal. In Florida, Hh2 Tapirus is known only from relatively incomplete specimens, but at least two species are represented, both of significantly smaller size than Tapirus webbi or Tapirus simpsoni. One appears to be the dwarf Tapirus polkensis (Olsen), previously known from the very late Hemphillian (Hh4) in Florida and the Hemphillian of Tennessee (referred specimens from Nebraska need to be reexamined). Previous interpretations that the age of T. polkensis is middle Miocene are incorrect; its chronologic range in Florida is Hh2 to Hh4 based on direct association with biochronologic indicator taxa such as Neohipparion eurystyle, Dinohippus mexicanus and Agriotherium schneideri. -

Honeymoon Island Beach Nourishment Field Trip, 2015, 37 P
Honeymoon Island Beach Nourishment Field Trip Southeastern Geological Society Guidebook No. 64 June 12-13, 2015 A Field Guide to Honeymoon Island Beach Nourishment Southeastern Geological Society Guidebook No. 64 Field Trip June 12-13, 2015 2015 SEGS OFFICERS President – Greg Mudd Vice President – Bryan Carrick Secretary – Samantha Andrews Treasurer – Harley Means Past President - John Herbert Guidebook Compiled and Edited by: Bryan Carrick, P.G., 2015 Published by: THE SOUTHEASTERN GEOLOGICAL SOCIETY P.O. Box 1636 Tallahassee, Florida 32302 Southeastern Geological Society Guidebook No. 64 June 12-13, 2015 TABLE OF CONTENTS INTRODUCTION AND ACKNOWLEDGMENTS by: Bryan Carrick, P.G. …............................................................................................... 2 HONEYMOON ISLAND BEACH RESTORATION PROJECT by: Brett D. Moore, P.E., Humiston & Moore Engineers .................................................. 3 ROSS/OSSI (ROSSI): A COASTAL MANAGEMENT TOOL FOR OFFSHORE SAND SOURCES by:Jennifer L. Coor1, Candace Beauvais2, Jase D. Ousley3................................................ 9 SEDIMENT ENGINEERING THRU DREDGING AND WITH NATURE (SETDWN) – FATE OF FINES IN THE DREDGING AND PLACEMENT PROCESS by:Coraggio K. Maglio1, Jase D. Ousley2, Jennifer L.Coor3.…..…………......................16 MODERN AND HISTORICAL MORPHODYNAMICS OF THE JOHN’S PASS - BLIND PASS DUAL-INLET SYSTEM, PINELLAS COUNTY, FLORIDA by: Mark H. Horwitz, University of South Florida........................................................... 23 INVERTEBRATE PALEONTOLOGY -

Miocene Paleontology and Stratigraphy of the Suwannee River Basin of North Florida and South Georgia
MIOCENE PALEONTOLOGY AND STRATIGRAPHY OF THE SUWANNEE RIVER BASIN OF NORTH FLORIDA AND SOUTH GEORGIA SOUTHEASTERN GEOLOGICAL SOCIETY Guidebook Number 30 October 7, 1989 MIOCENE PALEONTOLOGY AND STRATIGRAPHY OF THE SUWANNEE RIVER BASIN OF NORTH FLORIDA AND SOUTH GEORGIA Compiled and edit e d by GARY S . MORGAN GUIDEBOOK NUMBER 30 A Guidebook for the Annual Field Trip of the Southeastern Geological Society October 7, 1989 Published by the Southeastern Geological Society P. 0 . Box 1634 Tallahassee, Florida 32303 TABLE OF CONTENTS Map of field trip area ...... ... ................................... 1 Road log . ....................................... ..... ..... ... .... 2 Preface . .................. ....................................... 4 The lithostratigraphy of the sediments exposed along the Suwannee River in the vicinity of White Springs by Thomas M. scott ........................................... 6 Fossil invertebrates from the banks of the Suwannee River at White Springs, Florida by Roger W. Portell ...... ......................... ......... 14 Miocene vertebrate faunas from the Suwannee River Basin of North Florida and South Georgia by Gary s. Morgan .................................. ........ 2 6 Fossil sirenians from the Suwannee River, Florida and Georgia by Daryl P. Damning . .................................... .... 54 1 HAMIL TON CO. MAP OF FIELD TRIP AREA 2 ROAD LOG Total Mileage from Reference Points Mileage Last Point 0.0 0.0 Begin at Holiday Inn, Lake City, intersection of I-75 and US 90. 7.3 7.3 Pass under I-10. 12 . 6 5.3 Turn right (east) on SR 136. 15.8 3 . 2 SR 136 Bridge over Suwannee River. 16.0 0.2 Turn left (west) on us 41. 19 . 5 3 . 5 Turn right (northeast) on CR 137. 23.1 3.6 On right-main office of Occidental Chemical Corporation.