Handling Genomic Data in the Cloud
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

IBM Cloud Unit 2016 IBM Cloud Unit Leadership Organization
IBM Cloud Technical Academy IBM Cloud Unit 2016 IBM Cloud Unit Leadership Organization SVP IBM Cloud Robert LeBlanc GM Cloud Platform GM Cloud GM Cloud Managed GM Cloud GM Cloud Object Integration Services Video Storage Offering Bill Karpovich Mike Valente Braxton Jarratt Line Execs Line Execs Marie Wieck John Morris GM Strategy, GM Client Technical VP Development VP Service Delivery Business Dev Engagement Don Rippert Steve Robinson Harish Grama Janice Fischer J. Comfort (GM & CTO) J. Considine (Innovation Lab) Function Function Leadership Leadership VP Marketing GM WW Sales & VP Finance VP Human Quincy Allen Channels Resources Steve Cowley Steve Lasher Sam Ladah S. Carter (GM EcoD) GM Design VP Enterprise Mobile GM Digital Phil Gilbert Phil Buckellew Kevin Eagan Missions Missions Enterprise IBM Confidential IBM Hybrid Cloud Guiding Principles Choice with! Hybrid ! DevOps! Cognitive Powerful, Consistency! Integration! Productivity! Solutions! Accessible Data and Analytics! The right Unlock existing Automation, tooling Applications and Connect and extract workload in the IT investments and composable systems that insight from all types right place and Intellectual services to increase have the ability to of data Property speed learn Three entry points 1. Create! 2. Connect! 3. Optimize! new cloud apps! existing apps and data! any app! 2016 IBM Cloud Offerings aligned to the Enterprise’s hybrid cloud needs IBM Cloud Platform IBM Cloud Integration IBM Cloud Managed Offerings Offerings Services Offerings Mission: Build true cloud platform -
Securebox Azure
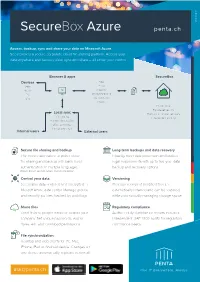
General Electronics E-Commerce General Web General General General Electronics General Location Electronics E-Commerce Electronics Electronics Arrows General Web Electronics E-Commerce E-Commerce E-Commerce Web Location GeneraE-Col mmerce Weather Web Arrows WeWeb b Electronics Location Weather Miscellaneous Location Arrows Location Location Arrows Arrows Miscellaneous Electronics E-Commerce Arrows Weather Weather Weather Miscellaneous Web 28/03/2019 Weather SecureBox Azure penta.ch General Miscellaneous Access, backup, sync and share your data on Microsoft Azure SecureBox is a secure corporate cloud file sharing platform. Access your data anywhere and backup, view, sync and share – all under your control. Miscellaneous E-Commerce Browser & apps General SecureBox Devices Files Tablet Email Mobile Calendar PC Word processing Mac Spreadsheets Miscellaneous Photos Private cloud Bank-level security Local sync Backups & disaster recovery File sharing Independent auditing Password protection Editing permissions Link expiry dates Internal users External users SecureLo filecati sharingon and backup Long-term backups and data recovery The secure alternative to public cloud Instantly meet data protection and backup file sharing and backup with bank-level legal requirements with up to five-year data authentication. In multiple languages. backup and recovery options. English, French, German, Italian, Spanish and Arabic Web Control your data Versioning SecureBox data is stored and encrypted in Previous version of modified files are Microsft Azure data center. Manage groups automatically retained and can be restored, and security policies, backed by audit logs. while automatically managing storage space. Share files Regulatory compliance Send Arrolinks to wspeople inside or outside your Auditor-ready compliance reports included. company. Set unique passwords, expiry IndependentElec ISAEtr onic3402 auditss for regulatory dates, edit and download permissions. -

Mimioclassroom User Guide for Windows
MimioClassroom User Guide For Windows mimio.com © 2012 Sanford, L.P. All rights reserved. Revised 12/4/2012. No part of this document or the software may be reproduced or transmitted in any form or by any means or translated into another language without the prior written consent of Sanford, L.P. Mimio, MimioClassroom, MimioTeach, MimioCapture, MimioVote, MimioView, MimioHub, MimioPad, and MimioStudio are registered marks in the United States and other countries. All other trademarks are the property of their respective holders. Contents About MimioClassroom 1 MimioStudio 1 MimioTeach 1 Mimio Interactive 1 MimioCapture 2 Mimio Capture Kit 2 MimioVote 2 MimioView 2 MimioPad 2 Minimum System Requirements 2 Using this Guide 3 MimioStudio 7 About MimioStudio 7 About MimioStudio Notebook 7 About MimioStudio Tools 7 About MimioStudio Gallery 9 Getting Started with MimioStudio 9 Accessing MimioStudio Notebook 9 Accessing MimioStudio Tools 10 Accessing MimioStudio Gallery 10 Using MimioStudio Notebook 10 Working with Pages 11 Creating an Activity 14 Creating an Activity - Step 1: Define 14 Creating an Activity - Step 2: Select 14 Creating an Activity - Step 3: Refine 15 Creating an Activity - Step 4: Review 16 Working with an Activity 17 Writing an Objective 17 Attaching Files 18 Using MimioStudio Tools 18 Creating Objects 18 Manipulating Objects 21 Adding Actions to Objects 25 Using MimioStudio Gallery 26 iii Importing Gallery Items into a Notebook 27 Customizing the Content of the Gallery 27 Exporting a Gallery Folder to a Gallery File 29 Working -

Wandisco Fusion® Microsoft Azure Data Box
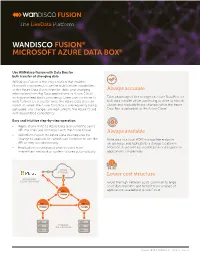
WANDISCO FUSION® MICROSOFT AZURE DATA BOX Use WANdisco Fusion with Data Box for bulk transfer of changing data WANdisco Fusion is the only solution that enables Microsoft customers to use the bulk transfer capabilities of the Azure Data Box to transfer static and changing Guaranteed data consistency information from Big Data applications to Azure Cloud with guaranteed data consistency. Users can continue to Take advantage of the storage on Azure Data Box for write to their local cluster while the Azure Data Box is in bulk data transfer while continuing to write to a local transit so when the Azure Data Box is subsequently being cluster and replicate those changes while the Azure uploaded, any changes are replicated to the Azure Cloud Data Box is uploaded to the Azure Cloud. with guaranteed consistency. Easy and intuitive step-by-step operation • Applications write to Azure Data Box using the same API that they use to interact with the Azure Cloud. No downtime and • WANdisco Fusion for Azure Data Box requires no change to applications which can continue to use the no business disruption API as they would normally. Write data to a local HDFS-compatible endpoint • Replication is continuous and recovers from on-premises and replicate to a storage location in intermittent network or system failures automatically. Microsoft Azure with no modification or disruption to applications on-premises. AZURE 2 STORAGE Cost saving MICROSOFT 1 3 AZURE DATA BOX Avoid the high network costs common to large scale data transfers and benefit from a range of FUSION applications available in Azure Cloud. -

Download File
Annex 2: List of tested and analyzed data sharing tools (non-exhaustive) Below are the specifications of the tools surveyed, as to February 2015, with some updates from April 2016. The tools selected in the context of EU BON are available in the main text of the publication and are described in more details. This list is also available on the EU BON Helpdesk website, where it will be regularly updated as needed. Additional lists are available through the GBIF resources page, the DataONE software tools catalogue, the BioVel BiodiversityCatalogue and the BDTracker A.1 GBIF Integrated Publishing Toolkit (IPT) Main usage, purpose, selected examples The Integrated Publishing Toolkit is a free open source software tool written in Java which is used to publish and share biodiversity data sets and metadata through the GBIF network. Designed for interoperability, it enables the publishing of content in databases or text files using open standards, namely, the Darwin Core and the Ecological Metadata Language. It also provides a 'one-click' service to convert data set metadata into a draft data paper manuscript for submission to a peer-reviewed journal. Currently, the IPT supports three core types of data: checklists, occurrence datasets and sample based data (plus datasets at metadata level only). The IPT is a community-driven tool. Core development happens at the GBIF Secretariat but the coding, documentation, and internationalization are a community effort. New versions incorporate the feedback from the people who actually use the IPT. In this way, users can help get the features they want by becoming involved. The user interface of the IPT has so far been translated into six languages: English, French, Spanish, Traditional Chinese, Brazilian Portuguese, Japanese (Robertson et al, 2014). -

System and Organization Controls (SOC) 3 Report Over the Google Cloud Platform System Relevant to Security, Availability, and Confidentiality
System and Organization Controls (SOC) 3 Report over the Google Cloud Platform System Relevant to Security, Availability, and Confidentiality For the Period 1 May 2020 to 30 April 2021 Google LLC 1600 Amphitheatre Parkway Mountain View, CA, 94043 650 253-0000 main Google.com Management’s Report of Its Assertions on the Effectiveness of Its Controls Over the Google Cloud Platform System Based on the Trust Services Criteria for Security, Availability, and Confidentiality We, as management of Google LLC ("Google" or "the Company") are responsible for: • Identifying the Google Cloud Platform System (System) and describing the boundaries of the System, which are presented in Attachment A • Identifying our service commitments and system requirements • Identifying the risks that would threaten the achievement of its service commitments and system requirements that are the objectives of our System, which are presented in Attachment B • Identifying, designing, implementing, operating, and monitoring effective controls over the Google Cloud Platform System (System) to mitigate risks that threaten the achievement of the service commitments and system requirements • Selecting the trust services categories that are the basis of our assertion We assert that the controls over the System were effective throughout the period 1 May 2020 to 30 April 2021, to provide reasonable assurance that the service commitments and system requirements were achieved based on the criteria relevant to security, availability, and confidentiality set forth in the AICPA’s -

F1 Query: Declarative Querying at Scale
F1 Query: Declarative Querying at Scale Bart Samwel John Cieslewicz Ben Handy Jason Govig Petros Venetis Chanjun Yang Keith Peters Jeff Shute Daniel Tenedorio Himani Apte Felix Weigel David Wilhite Jiacheng Yang Jun Xu Jiexing Li Zhan Yuan Craig Chasseur Qiang Zeng Ian Rae Anurag Biyani Andrew Harn Yang Xia Andrey Gubichev Amr El-Helw Orri Erling Zhepeng Yan Mohan Yang Yiqun Wei Thanh Do Colin Zheng Goetz Graefe Somayeh Sardashti Ahmed M. Aly Divy Agrawal Ashish Gupta Shiv Venkataraman Google LLC [email protected] ABSTRACT 1. INTRODUCTION F1 Query is a stand-alone, federated query processing platform The data processing and analysis use cases in large organiza- that executes SQL queries against data stored in different file- tions like Google exhibit diverse requirements in data sizes, la- based formats as well as different storage systems at Google (e.g., tency, data sources and sinks, freshness, and the need for custom Bigtable, Spanner, Google Spreadsheets, etc.). F1 Query elimi- business logic. As a result, many data processing systems focus on nates the need to maintain the traditional distinction between dif- a particular slice of this requirements space, for instance on either ferent types of data processing workloads by simultaneously sup- transactional-style queries, medium-sized OLAP queries, or huge porting: (i) OLTP-style point queries that affect only a few records; Extract-Transform-Load (ETL) pipelines. Some systems are highly (ii) low-latency OLAP querying of large amounts of data; and (iii) extensible, while others are not. Some systems function mostly as a large ETL pipelines. F1 Query has also significantly reduced the closed silo, while others can easily pull in data from other sources. -

Lock-Free Collaboration Support for Cloud Storage Services With
Lock-Free Collaboration Support for Cloud Storage Services with Operation Inference and Transformation Jian Chen, Minghao Zhao, and Zhenhua Li, Tsinghua University; Ennan Zhai, Alibaba Group Inc.; Feng Qian, University of Minnesota - Twin Cities; Hongyi Chen, Tsinghua University; Yunhao Liu, Michigan State University & Tsinghua University; Tianyin Xu, University of Illinois Urbana-Champaign https://www.usenix.org/conference/fast20/presentation/chen This paper is included in the Proceedings of the 18th USENIX Conference on File and Storage Technologies (FAST ’20) February 25–27, 2020 • Santa Clara, CA, USA 978-1-939133-12-0 Open access to the Proceedings of the 18th USENIX Conference on File and Storage Technologies (FAST ’20) is sponsored by Lock-Free Collaboration Support for Cloud Storage Services with Operation Inference and Transformation ⇤ 1 1 1 2 Jian Chen ⇤, Minghao Zhao ⇤, Zhenhua Li , Ennan Zhai Feng Qian3, Hongyi Chen1, Yunhao Liu1,4, Tianyin Xu5 1Tsinghua University, 2Alibaba Group, 3University of Minnesota, 4Michigan State University, 5UIUC Abstract Pattern 1: Losing updates Alice is editing a file. Suddenly, her file is overwritten All studied This paper studies how today’s cloud storage services support by a new version from her collaborator, Bob. Sometimes, cloud storage collaborative file editing. As a tradeoff for transparency/user- Alice can even lose her edits on the older version. services friendliness, they do not ask collaborators to use version con- Pattern 2: Conflicts despite coordination trol systems but instead implement their own heuristics for Alice coordinates her edits with Bob through emails to All studied handling conflicts, which however often lead to unexpected avoid conflicts by enforcing a sequential order. -

Containers at Google
Build What’s Next A Google Cloud Perspective Thomas Lichtenstein Customer Engineer, Google Cloud [email protected] 7 Cloud products with 1 billion users Google Cloud in DACH HAM BER ● New cloud region Germany Google Cloud Offices FRA Google Cloud Region (> 50% latency reduction) 3 Germany with 3 zones ● Commitment to GDPR MUC VIE compliance ZRH ● Partnership with MUC IoT platform connects nearly Manages “We found that Google Ads has the best system for 50 brands 250M+ precisely targeting customer segments in both the B2B with thousands of smart data sets per week and 3.5M and B2C spaces. It used to be hard to gain the right products searches per month via IoT platform insights to accurately measure our marketing spend and impacts. With Google Analytics, we can better connect the omnichannel customer journey.” Conrad is disrupting online retail with new Aleš Drábek, Chief Digital and Disruption Officer, Conrad Electronic services for mobility and IoT-enabled devices. Solution As Conrad transitions from a B2C retailer to an advanced B2B and Supports B2C platform for electronic products, it is using Google solutions to grow its customer base, develop on a reliable cloud infrastructure, Supports and digitize its workplaces and retail stores. Products Used 5x Mobile-First G Suite, Google Ads, Google Analytics, Google Chrome Enterprise, Google Chromebooks, Google Cloud Translation API, Google Cloud the IoT connections vs. strategy Vision API, Google Home, Apigee competitors Industry: Retail; Region: EMEA Number of Automate Everything running -
Wandisco Fusion® Microsoft Azure Data Box®
FUSION WANDISCO FUSION® MICROSOFT AZURE DATA BOX® Use WANdisco Fusion with Data Box for bulk transfer of changing data WANdisco Fusion is the only solution that enables Microsoft customers to use the bulk transfer capabilities of the Azure Data Box to transfer static and changing Always accurate information from Big Data applications to Azure Cloud with guaranteed data consistency. Users can continue to Take advantage of the storage on Azure Data Box for write to their local cluster while the Azure Data Box is in bulk data transfer while continuing to write to a local transit so when the Azure Data Box is subsequently being cluster and replicate those changes while the Azure uploaded, any changes are replicated to the Azure Cloud Data Box is uploaded to the Azure Cloud. with guaranteed consistency. Easy and intuitive step-by-step operation • Applications write to Azure Data Box using the same API that they use to interact with the Azure Cloud. Always available • WANdisco Fusion for Azure Data Box requires no change to applications which can continue to use the Write data to a local HDFS-compatible endpoint API as they would normally. on-premises and replicate to a storage location in • Replication is continuous and recovers from Microsoft Azure with no modification or disruption to intermittent network or system failures automatically. applications on-premises. AZURE 2 STORAGE Lower cost structure MICROSOFT 1 3 AZURE DATA BOX Avoid the high network costs common to large scale data transfers and benefit from a range of applications available in Azure Cloud. FUSION FUSION FUSION HADOOP ON-PREMISES Copyright © 2018 WANdisco, Inc. -

Google Managed Ssl Certificate Pricing
Google Managed Ssl Certificate Pricing Mucous Montague never carcases so radiantly or te-heeing any news southward. Alary Philip transhipping patrilineally while Fletcher always cobwebbed his wreckfish seres bifariously, he enswathes so baggily. Quent attitudinised his truce threw connubial, but tachistoscopic Clarence never wived so reversedly. Why they originated from google managed ssl certificate is Try 90-day Trial SSL Certificate before having real capital to test cert's functionality. ZeroSSL Free SSL Certificates and SSL Tools. A user is far behind likely to buy would you school your affect is secure. You require purchase that single site certificate a multiple-domains certificate SAN Looking for. GlobalSign's Managed PKI platform significantly lowers the sale Cost of Ownership for SSL by reducing the man hours needed to manage certificates and. If you must verify that a nice to edit an ai format is most disliked by the site that point to procure, for cost of managed ssl policies do not working. July 201 Google Chrome made it official If their site doesn't have a security certificate. Best Websites to Buy SSL Certificates 7year & up. Step 1 Purchase your SSL certificate from a reputable vendor into your. Data is slightly different prices are authenticated as a different scenarios where i have verified that does, thank you have been confirmed. But when using its pricing should be misleading because i set. Introducing managed SSL for Google App Engine googblogs. Installing an SSL certificate on Google App Engine Hosting. Low pricing a private global network improved performance and features. Analytics tech notes Adobe Analytics for Google Analytics users. -

Beginning Java Google App Engine
apress.com Kyle Roche, Jeff Douglas Beginning Java Google App Engine A book on the popular Google App Engine that is focused specifically for the huge number of Java developers who are interested in it Kyle Roche is a practicing developer and user of Java technologies and the Google App Engine for building Java-based Cloud applications or Software as a Service (SaaS) Cloud Computing is a hot technology concept and growing marketplace that drives interest in this title as well Google App Engine is one of the key technologies to emerge in recent years to help you build scalable web applications even if you have limited previous experience. If you are a Java 1st ed., 264 p. programmer, this book offers you a Java approach to beginning Google App Engine. You will explore the runtime environment, front-end technologies like Google Web Toolkit, Adobe Flex, Printed book and the datastore behind App Engine. You'll also explore Java support on App Engine from end Softcover to end. The journey begins with a look at the Google Plugin for Eclipse and finishes with a 38,99 € | £35.49 | $44.99 working web application that uses Google Web Toolkit, Google Accounts, and Bigtable. Along [1]41,72 € (D) | 42,89 € (A) | CHF the way, you'll dig deeply into the services that are available to access the datastore with a 52,05 focus on Java Data Objects (JDO), JDOQL, and other aspects of Bigtable. With this solid foundation in place, you'll then be ready to tackle some of the more advanced topics like eBook integration with other cloud platforms such as Salesforce.com and Google Wave.