Kavalactones and Flavokavains in Piper Methysticum (Kava)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
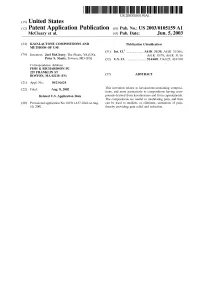
(12) Patent Application Publication (10) Pub. No.: US 2003/0105159 A1 Mccleary Et Al
US 200301 05159A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0105159 A1 McCleary et al. (43) Pub. Date: Jun. 5, 2003 (54) KAVALACTONE COMPOSITIONS AND Publication Classification METHODS OF USE (51) Int. Cl." ....................... A61K 31/35; A61K 31/366; (76) Inventors: Joel McCleary, The Plains, VA (US); A61K 35/78; A61K 31/16 Peter S. Staats, Towson, MD (US) (52) U.S. Cl. ........................... 514/460; 514/625; 424/760 Correspondence Address: FISH & RICHARDSON PC 225 FRANKLIN ST BOSTON, MA 02110 (US) (57) ABSTRACT (21) Appl. No.: 10/214,624 (22) Filed: Aug. 8, 2002 This invention relates tO kavalactone-containing composi tions, and more particularly to compositions having com Related U.S. Application Data pounds derived from kavalactones and from capsaicinoids. The compositions are useful in modulating pain, and thus (60) Provisional application No. 60/311,437, filed on Aug. can be used to mediate, or eliminate, Sensations of pain, 10, 2001. thereby providing pain relief and reduction. US 2003/0105159 A1 Jun. 5, 2003 KAVALACTONE COMPOSITIONS AND METHODS 0006. In one embodiment, the invention relates to an OF USE analgesic topical composition having: (a) a kavalactone; (b) capsaicinoid or Synthetic derivatives thereof; and (c) a CROSS-REFERENCE TO RELATED pharmaceutically acceptable carrier; wherein the weight APPLICATIONS ratio of(a):(b) is from 5000:1 to 1:2 (e.g., 800:1 to 1:1; 500:1 to 5:1). In other aspects, the composition includes an effec 0001. This application claims benefit of U.S. application tive amount of kavalactones, active kavalactones, or capsai Ser. -

(12) Patent Application Publication (10) Pub. No.: US 2016/017.4603 A1 Abayarathna Et Al
US 2016O174603A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/017.4603 A1 Abayarathna et al. (43) Pub. Date: Jun. 23, 2016 (54) ELECTRONIC VAPORLIQUID (52) U.S. Cl. COMPOSITION AND METHOD OF USE CPC ................. A24B 15/16 (2013.01); A24B 15/18 (2013.01); A24F 47/002 (2013.01) (71) Applicants: Sahan Abayarathna, Missouri City, TX 57 ABSTRACT (US); Michael Jaehne, Missouri CIty, An(57) e-liquid for use in electronic cigarettes which utilizes- a TX (US) vaporizing base (either propylene glycol, vegetable glycerin, (72) Inventors: Sahan Abayarathna, MissOU1 City,- 0 TX generallyor mixture at of a 0.001 the two) g-2.0 mixed g per with 1 mL an ratio. herbal The powder herbal extract TX(US); (US) Michael Jaehne, Missouri CIty, can be any of the following:- - - Kanna (Sceletium tortuosum), Blue lotus (Nymphaea caerulea), Salvia (Salvia divinorum), Salvia eivinorm, Kratom (Mitragyna speciosa), Celandine (21) Appl. No.: 14/581,179 poppy (Stylophorum diphyllum), Mugwort (Artemisia), Coltsfoot leaf (Tussilago farfara), California poppy (Eschscholzia Californica), Sinicuichi (Heimia Salicifolia), (22) Filed: Dec. 23, 2014 St. John's Wort (Hypericum perforatum), Yerba lenna yesca A rtemisia scoparia), CaleaCal Zacatechichihichi (Calea(Cal termifolia), Leonurus Sibericus (Leonurus Sibiricus), Wild dagga (Leono Publication Classification tis leonurus), Klip dagga (Leonotis nepetifolia), Damiana (Turnera diffiisa), Kava (Piper methysticum), Scotch broom (51) Int. Cl. tops (Cytisus scoparius), Valarien (Valeriana officinalis), A24B 15/16 (2006.01) Indian warrior (Pedicularis densiflora), Wild lettuce (Lactuca A24F 47/00 (2006.01) virosa), Skullcap (Scutellaria lateriflora), Red Clover (Trifo A24B I5/8 (2006.01) lium pretense), and/or combinations therein. -

(12) United States Patent (10) Patent No.: US 9.421,180 B2 Zielinski Et Al
USOO9421 180B2 (12) United States Patent (10) Patent No.: US 9.421,180 B2 Zielinski et al. (45) Date of Patent: Aug. 23, 2016 (54) ANTIOXIDANT COMPOSITIONS FOR 6,203,817 B1 3/2001 Cormier et al. .............. 424/464 TREATMENT OF INFLAMMATION OR 6,323,232 B1 1 1/2001 Keet al. ............ ... 514,408 6,521,668 B2 2/2003 Anderson et al. ..... 514f679 OXIDATIVE DAMAGE 6,572,882 B1 6/2003 Vercauteren et al. ........ 424/451 6,805,873 B2 10/2004 Gaudout et al. ....... ... 424/401 (71) Applicant: Perio Sciences, LLC, Dallas, TX (US) 7,041,322 B2 5/2006 Gaudout et al. .............. 424/765 7,179,841 B2 2/2007 Zielinski et al. .. ... 514,474 (72) Inventors: Jan Zielinski, Vista, CA (US); Thomas 2003/0069302 A1 4/2003 Zielinski ........ ... 514,452 Russell Moon, Dallas, TX (US); 2004/0037860 A1 2/2004 Maillon ...... ... 424/401 Edward P. Allen, Dallas, TX (US) 2004/0091589 A1 5, 2004 Roy et al. ... 426,265 s s 2004/0224004 A1 1 1/2004 Zielinski ..... ... 424/442 2005/0032882 A1 2/2005 Chen ............................. 514,456 (73) Assignee: Perio Sciences, LLC, Dallas, TX (US) 2005, 0137205 A1 6, 2005 Van Breen ..... 514,252.12 2005. O154054 A1 7/2005 Zielinski et al. ............. 514,474 (*) Notice: Subject to any disclaimer, the term of this 2005/0271692 Al 12/2005 Gervasio-Nugent patent is extended or adjusted under 35 et al. ............................. 424/401 2006/0173065 A1 8/2006 BeZwada ...................... 514,419 U.S.C. 154(b) by 19 days. 2006/O193790 A1 8/2006 Doyle et al. -

Herbal Insomnia Medications That Target Gabaergic Systems: a Review of the Psychopharmacological Evidence
Send Orders for Reprints to [email protected] Current Neuropharmacology, 2014, 12, 000-000 1 Herbal Insomnia Medications that Target GABAergic Systems: A Review of the Psychopharmacological Evidence Yuan Shia, Jing-Wen Donga, Jiang-He Zhaob, Li-Na Tanga and Jian-Jun Zhanga,* aState Key Laboratory of Bioactive Substance and Function of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, P.R. China; bDepartment of Pharmacology, School of Marine, Shandong University, Weihai, P.R. China Abstract: Insomnia is a common sleep disorder which is prevalent in women and the elderly. Current insomnia drugs mainly target the -aminobutyric acid (GABA) receptor, melatonin receptor, histamine receptor, orexin, and serotonin receptor. GABAA receptor modulators are ordinarily used to manage insomnia, but they are known to affect sleep maintenance, including residual effects, tolerance, and dependence. In an effort to discover new drugs that relieve insomnia symptoms while avoiding side effects, numerous studies focusing on the neurotransmitter GABA and herbal medicines have been conducted. Traditional herbal medicines, such as Piper methysticum and the seed of Zizyphus jujuba Mill var. spinosa, have been widely reported to improve sleep and other mental disorders. These herbal medicines have been applied for many years in folk medicine, and extracts of these medicines have been used to study their pharmacological actions and mechanisms. Although effective and relatively safe, natural plant products have some side effects, such as hepatotoxicity and skin reactions effects of Piper methysticum. In addition, there are insufficient evidences to certify the safety of most traditional herbal medicine. In this review, we provide an overview of the current state of knowledge regarding a variety of natural plant products that are commonly used to treat insomnia to facilitate future studies. -

Herbal Medicines in Pregnancy and Lactation : an Evidence-Based
00 Prelims 1410 10/25/05 2:13 PM Page i Herbal Medicines in Pregnancy and Lactation An Evidence-Based Approach Edward Mills DPh MSc (Oxon) Director, Division of Clinical Epidemiology Canadian College of Naturopathic Medicine North York, Ontario, Canada Jean-Jacques Duguoa MSc (cand.) ND Naturopathic Doctor Toronto Western Hospital Assistant Professor Division of Clinical Epidemiology Canadian College of Naturopathic Medicine North York, Ontario, Canada Dan Perri BScPharm MD MSc Clinical Pharmacology Fellow University of Toronto Toronto, Ontario, Canada Gideon Koren MD FACMT FRCP Director of Motherisk Professor of Medicine, Pediatrics and Pharmacology University of Toronto Toronto, Ontario, Canada With a contribution from Paul Richard Saunders PhD ND DHANP 00 Prelims 1410 10/25/05 2:13 PM Page ii © 2006 Taylor & Francis Medical, an imprint of the Taylor & Francis Group First published in the United Kingdom in 2006 by Taylor & Francis Medical, an imprint of the Taylor & Francis Group, 2 Park Square, Milton Park, Abingdon, Oxon OX14 4RN Tel.: ϩ44 (0)20 7017 6000 Fax.: ϩ44 (0)20 7017 6699 E-mail: [email protected] Website: www.tandf.co.uk/medicine All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or trans- mitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior permission of the publisher or in accordance with the provisions of the Copyright, Designs and Patents Act 1988 or under the terms of any licence permitting limited copying issued by the Copyright Licensing Agency, 90 Tottenham Court Road, London W1P 0LP. -

NIH Public Access Author Manuscript Pharmacol Ther
NIH Public Access Author Manuscript Pharmacol Ther. Author manuscript; available in PMC 2010 August 1. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Pharmacol Ther. 2009 August ; 123(2): 239±254. doi:10.1016/j.pharmthera.2009.04.002. Ethnobotany as a Pharmacological Research Tool and Recent Developments in CNS-active Natural Products from Ethnobotanical Sources Will C. McClatcheya,*, Gail B. Mahadyb, Bradley C. Bennettc, Laura Shielsa, and Valentina Savod a Department of Botany, University of Hawaìi at Manoa, Honolulu, HI 96822, U.S.A b Department of Pharmacy Practice, University of Illinois at Chicago, Chicago, IL 60612, U.S.A c Department of Biological Sciences, Florida International University, Miami, FL 33199, U.S.A d Dipartimento di Biologia dì Roma Trè, Viale Marconi, 446, 00146, Rome, Italy Abstract The science of ethnobotany is reviewed in light of its multidisciplinary contributions to natural product research for the development of pharmaceuticals and pharmacological tools. Some of the issues reviewed involve ethical and cultural perspectives of healthcare and medicinal plants. While these are not usually part of the discussion of pharmacology, cultural concerns potentially provide both challenges and insight for field and laboratory researchers. Plant evolutionary issues are also considered as they relate to development of plant chemistry and accessing this through ethnobotanical methods. The discussion includes presentation of a range of CNS-active medicinal plants that have been recently examined in the field, laboratory and/or clinic. Each of these plants is used to illustrate one or more aspects about the valuable roles of ethnobotany in pharmacological research. -

Review Article Small Molecules from Nature Targeting G-Protein Coupled Cannabinoid Receptors: Potential Leads for Drug Discovery and Development
Hindawi Publishing Corporation Evidence-Based Complementary and Alternative Medicine Volume 2015, Article ID 238482, 26 pages http://dx.doi.org/10.1155/2015/238482 Review Article Small Molecules from Nature Targeting G-Protein Coupled Cannabinoid Receptors: Potential Leads for Drug Discovery and Development Charu Sharma,1 Bassem Sadek,2 Sameer N. Goyal,3 Satyesh Sinha,4 Mohammad Amjad Kamal,5,6 and Shreesh Ojha2 1 Department of Internal Medicine, College of Medicine and Health Sciences, United Arab Emirates University, P.O. Box 17666, Al Ain, Abu Dhabi, UAE 2Department of Pharmacology and Therapeutics, College of Medicine and Health Sciences, United Arab Emirates University, P.O. Box 17666, Al Ain, Abu Dhabi, UAE 3DepartmentofPharmacology,R.C.PatelInstituteofPharmaceuticalEducation&Research,Shirpur,Mahrastra425405,India 4Department of Internal Medicine, College of Medicine, Charles R. Drew University of Medicine and Science, Los Angeles, CA 90059, USA 5King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia 6Enzymoics, 7 Peterlee Place, Hebersham, NSW 2770, Australia Correspondence should be addressed to Shreesh Ojha; [email protected] Received 24 April 2015; Accepted 24 August 2015 Academic Editor: Ki-Wan Oh Copyright © 2015 Charu Sharma et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The cannabinoid molecules are derived from Cannabis sativa plant which acts on the cannabinoid receptors types 1 and 2 (CB1 and CB2) which have been explored as potential therapeutic targets for drug discovery and development. Currently, there are 9 numerous cannabinoid based synthetic drugs used in clinical practice like the popular ones such as nabilone, dronabinol, and Δ - tetrahydrocannabinol mediates its action through CB1/CB2 receptors. -

Plant-Based Medicines for Anxiety Disorders, Part 2: a Review of Clinical Studies with Supporting Preclinical Evidence
CNS Drugs 2013; 24 (5) Review Article Running Header: Plant-Based Anxiolytic Psychopharmacology Plant-Based Medicines for Anxiety Disorders, Part 2: A Review of Clinical Studies with Supporting Preclinical Evidence Jerome Sarris,1,2 Erica McIntyre3 and David A. Camfield2 1 Department of Psychiatry, Faculty of Medicine, University of Melbourne, Richmond, VIC, Australia 2 The Centre for Human Psychopharmacology, Swinburne University of Technology, Melbourne, VIC, Australia 3 School of Psychology, Charles Sturt University, Wagga Wagga, NSW, Australia Correspondence: Jerome Sarris, Department of Psychiatry and The Melbourne Clinic, University of Melbourne, 2 Salisbury Street, Richmond, VIC 3121, Australia. Email: [email protected], Acknowledgements Dr Jerome Sarris is funded by an Australian National Health & Medical Research Council fellowship (NHMRC funding ID 628875), in a strategic partnership with The University of Melbourne, The Centre for Human Psychopharmacology at the Swinburne University of Technology. Jerome Sarris, Erica McIntyre and David A. Camfield have no conflicts of interest that are directly relevant to the content of this article. 1 Abstract Research in the area of herbal psychopharmacology has revealed a variety of promising medicines that may provide benefit in the treatment of general anxiety and specific anxiety disorders. However, a comprehensive review of plant-based anxiolytics has been absent to date. Thus, our aim was to provide a comprehensive narrative review of plant-based medicines that have clinical and/or preclinical evidence of anxiolytic activity. We present the article in two parts. In part one, we reviewed herbal medicines for which only preclinical investigations for anxiolytic activity have been performed. In this current article (part two), we review herbal medicines for which there have been both preclinical and clinical investigations for anxiolytic activity. -

Kava Kava Extract Is Available from Ashland Chemical Co., Mini Star International, Inc., and QBI (Quality Botanical Ingredients, Inc.)
SUMMARY OF DATA FOR CHEMICAL SELECTION Kava Kava 9000-38-8; 84696-40-2 November 1998 TABLE OF CONTENTS Basis for Nomination Chemical Identification Production Information Use Pattern Human Exposure Regulatory Status Evidence for Possible Carcinogenic Activity Human Data Animal Data Metabolism Other Biological Effects Structure-Activity Relationships References BASIS OF NOMINATION TO THE CSWG Kava kava is brought to the attention of the CSWG because it is a rapidly growing, highly used dietary supplement introduced into the mainstream U.S. market relatively recently. Through this use, millions of consumers using antianxiety preparations are potentially exposed to kava kava. A traditional beverage of various Pacific Basin countries, kava clearly has psychoactive properties. The effects of its long-term consumption have not been documented adequately; preliminary studies suggest possibly serious organ system effects. The potential carcinogenicity of kava and its principal constituents are unknown. INPUT FROM GOVERNMENT AGENCIES/INDUSTRY The U.S. Pharmacopeia is in the process of reviewing kava kava. No decision on preparation of a monograph has been made. SELECTION STATUS ACTION BY CSWG: 12/14/98 Studies requested: - Toxicological evaluation, to include studies of reproductive toxicity and neurotoxicity - Genotoxicity Priority: High Rationale/Remarks: - Significant human exposure - Leading dietary supplement with rapidly growing use - Concern that kava has been promoted as a substitute for ritilin in children - Test extract standardized to 30 percent kavalactones - NCI is conducting studies in Salmonella typhimurium CHEMICAL IDENTIFICATION CAS Registry Number: 9000-38-8 Kava-kava resin (8CI) Chemical Abstract Service Name: 84696-40-2 CAS Registry Number: Pepper (Piper), P. methysticum, ext. Chemical Abstract Service Name: Extract of kava; kava extract; Piper Synonyms and Trade Names: methisticum extract Description: The tropical shrub Piper methysticum is widely cultivated in the South Pacific. -

Article – – = = = = C7-C8 – – – – Is Known to = = C5-C6
J. Braz. Chem. Soc., Vol. 20, No. 9, 1687-1697, 2009. Printed in Brazil - ©2009 Sociedade Brasileira de Química 0103 - 5053 $6.00+0.00 Article Synthesis of Novel Kavain-like Derivatives and Evaluation of their Cytotoxic Activity Patricia de A. Amaral,a,b Julien Petrignet,c Nicolas Gouault,b Taciane Agustini,a Françoise Lohézic-Ledévéhat,b Alexandre Cariou,b René Grée,c Vera L. Eifler-Lima*,a and Michèle Davidb aLaboratório de Síntese Orgânica Medicinal, Faculdade de Farmácia, Universidade Federal do Rio Grande do Sul, 90610-000 Porto Alegre-RS, Brazil bLaboratoire de Chimie Therapeutique, UPRES 4090, Université de Rennes 1, Rennes, France cLaboratoire de Chimie et Photonique Moléculaires, CNRS UMR 6510, Université de Rennes 1, Rennes, France Reações de acoplamento do tipo Heck, Sonogashira-Hagihara, Suzuki-Miyaura e reação de aldolisação catalizadas por metal foram utilizadas para a obtenção de três séries de d-valerolactonas substituídas em posições 3, 4, 5 e 6 do anel lactônico. As 26 d-valerolactonas sintetizadas foram testadas contra três linhagens celulares e cinco delas exibiram uma moderada atividade citotóxica. Palladium-catalyzed cross coupling reactions (Sonogashira-Hagihara, Suzuki-Miyaura, and Heck) coupling and nickel hydride-mediated tandem isomerization aldolisation have been used for the synthesis of three series of d-valerolactones substituted in positions 3, 4, 5 and 6 of the lactone ring. The 26 kavaïn-like derivatives were tested against three cell lines and five of them exhibited a weak cytotoxic activity. Keywords: -

Phytochem Referenzsubstanzen
High pure reference substances Phytochem Hochreine Standardsubstanzen for research and quality für Forschung und management Referenzsubstanzen Qualitätssicherung Nummer Name Synonym CAS FW Formel Literatur 01.286. ABIETIC ACID Sylvic acid [514-10-3] 302.46 C20H30O2 01.030. L-ABRINE N-a-Methyl-L-tryptophan [526-31-8] 218.26 C12H14N2O2 Merck Index 11,5 01.031. (+)-ABSCISIC ACID [21293-29-8] 264.33 C15H20O4 Merck Index 11,6 01.032. (+/-)-ABSCISIC ACID ABA; Dormin [14375-45-2] 264.33 C15H20O4 Merck Index 11,6 01.002. ABSINTHIN Absinthiin, Absynthin [1362-42-1] 496,64 C30H40O6 Merck Index 12,8 01.033. ACACETIN 5,7-Dihydroxy-4'-methoxyflavone; Linarigenin [480-44-4] 284.28 C16H12O5 Merck Index 11,9 01.287. ACACETIN Apigenin-4´methylester [480-44-4] 284.28 C16H12O5 01.034. ACACETIN-7-NEOHESPERIDOSIDE Fortunellin [20633-93-6] 610.60 C28H32O14 01.035. ACACETIN-7-RUTINOSIDE Linarin [480-36-4] 592.57 C28H32O14 Merck Index 11,5376 01.036. 2-ACETAMIDO-2-DEOXY-1,3,4,6-TETRA-O- a-D-Glucosamine pentaacetate 389.37 C16H23NO10 ACETYL-a-D-GLUCOPYRANOSE 01.037. 2-ACETAMIDO-2-DEOXY-1,3,4,6-TETRA-O- b-D-Glucosamine pentaacetate [7772-79-4] 389.37 C16H23NO10 ACETYL-b-D-GLUCOPYRANOSE> 01.038. 2-ACETAMIDO-2-DEOXY-3,4,6-TRI-O-ACETYL- Acetochloro-a-D-glucosamine [3068-34-6] 365.77 C14H20ClNO8 a-D-GLUCOPYRANOSYLCHLORIDE - 1 - High pure reference substances Phytochem Hochreine Standardsubstanzen for research and quality für Forschung und management Referenzsubstanzen Qualitätssicherung Nummer Name Synonym CAS FW Formel Literatur 01.039. -

(12) United States Patent (10) Patent No.: US 6,746,695 B1 Martin Et Al
USOO6746695B1 (12) United States Patent (10) Patent No.: US 6,746,695 B1 Martin et al. (45) Date of Patent: Jun. 8, 2004 (54) PHARMACEUTICAL PREPARATIONS OF Abad, MJ et al., Anti-inflammatory activity of some medici BOACTIVE SUBSTANCES EXTRACTED nal plant extracts from Venezuela. J. Ethnopharmacol1996 FROM NATURAL SOURCES Dec;55(1):63–8. Alarcon-Aguilara, FJ, et al., Study of the anti-hyperglyce (75) Inventors: Michael Z. Martin, Laupahoehoe, HI mic effect of plants used as antidiabetics. J. Ethnopharmacol (US); Mehdi Ashraf-Khorassani, Blacksburg, VA (US); Larry Taylor, 612:101-110 (1998). Blacksburg, VA (US) Almeida CE et al., Analysis of anti-diarrheic effect of plants used in popular medicine. Rev Saude Publica 1995 (73) Assignees: Armadillo Pharmaceuticals, Inc., Dec;29(6):428-33. Armocas, CA (US); Virginia Tech. Alves KB, et al., Inhibition of aminopeptidase activity by Intellectual Properties, Inc., aromatic and other cyclic compounds. Braz, J Med Biol Res. Blackburg, VA (US) 1992:25(11):1103–6. (*) Notice: Subject to any disclaimer, the term of this Anesini C, et al., Screening of plants used in Argentine folk patent is extended or adjusted under 35 medicine for anti-microbial activity. J Ethnopharmacol. U.S.C. 154(b) by 0 days. 1993 Jun;39(2):119–28. Arletti, R, et al., Stimulating property of Turnera diffusa and Pfafia paniculata eXtracts on the Sexual behavior of male (21) Appl. No.: 09/578,849 rats, Psychopharmacology (Berl). 1999 Mar;143(1): 15-9. (22) Filed: May 26, 2000 Auterhoff, Het al., Constituents of the drug Damiana. Arch Related U.S.