Mobilization Failure in Hematopoietic Stem Cell Transplantation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Therapeutic Class Overview Colony Stimulating Factors
Therapeutic Class Overview Colony Stimulating Factors Therapeutic Class Overview/Summary: This review will focus on the granulocyte colony stimulating factors (G-CSFs) and granulocyte- macrophage colony stimulating factors (GM-CSFs).1-5 Colony-stimulating factors (CSFs) fall under the naturally occurring glycoprotein cytokines, one of the main groups of immunomodulators.6 In general, these proteins are vital to the proliferation and differentiation of hematopoietic progenitor cells.6-8 The G- CSFs commercially available in the United States include pegfilgrastim (Neulasta®), filgrastim (Neupogen®), filgrastim-sndz (Zarxio®), and tbo-filgrastim (Granix®). While filgrastim-sndz and tbo- filgrastim are the same recombinant human G-CSF as filgrastim, only filgrastim-sndz is considered a biosimilar drug as it was approved through the biosimilar pathway. At the time tbo-filgrastim was approved, a regulatory pathway for biosimilar drugs had not yet been established in the United States and tbo-filgrastim was filed under its own Biologic License Application.9 Only one GM-CSF is currently available, sargramostim (Leukine). These agents are Food and Drug Administration (FDA)-approved for a variety of conditions relating to neutropenia or for the collection of hematopoietic progenitor cells for collection by leukapheresis.1-5 Due to the pathway taken, tbo-filgrastim does not share all of the same indications as filgrastim and these two products are not interchangeable. It is important to note that although filgrastim-sndz is a biosimilar product, and it was approved with all the same indications as filgrastim at the time, filgrastim has since received FDA-approval for an additional indication that filgrastim-sndz does not have, to increase survival in patients with acute exposure to myelosuppressive doses of radiation.1-3A complete list of indications for each agent can be found in Table 1. -

Ontario Drug Benefit Formulary Edition 43
Ministry of Health and Long-Term Care Ontario Drug Benefit Formulary/Comparative Drug Index Edition 43 Drug Programs Policy and Strategy Branch Ontario Public Drug Programs Ministry of Health and Long-Term Care Effective February 28, 2018 Visit Formulary Downloads: Edition 43 Table of Contents Part I Introduction ....................................................................................................... I.1 Part II Preamble .......................................................................................................... II.1 Part III-A Benefits List ........................................................................................... III-A.1 Part III-B Off-Formulary Interchangeable Drugs (OFI) ........................................ III-B.1 Part IV Section Currently Not In Use ......................................................................... IV Part V Index of Pharmacologic-Therapeutic Classification .................................... V.1 Part VI-A Facilitated Access - HIV/AIDS .............................................................. VI-A.1 Part VI-B Facilitated Access - Palliative Care ..................................................... VI-B.1 Part VI-C Temporary Facilitated Access - Rheumatology ................................. VI-C.1 Part VII Trillium Drug Program ................................................................................ VII.1 Part VIII Exceptional Access Program (EAP) ........................................................ VIII.1 Part IX-A Nutrition Products ................................................................................ -

The Act of Controlling Adult Stem Cell Dynamics: Insights from Animal Models
biomolecules Review The Act of Controlling Adult Stem Cell Dynamics: Insights from Animal Models Meera Krishnan 1, Sahil Kumar 1, Luis Johnson Kangale 2,3 , Eric Ghigo 3,4 and Prasad Abnave 1,* 1 Regional Centre for Biotechnology, NCR Biotech Science Cluster, 3rd Milestone, Gurgaon-Faridabad Ex-pressway, Faridabad 121001, India; [email protected] (M.K.); [email protected] (S.K.) 2 IRD, AP-HM, SSA, VITROME, Aix-Marseille University, 13385 Marseille, France; [email protected] 3 Institut Hospitalo Universitaire Méditerranée Infection, 13385 Marseille, France; [email protected] 4 TechnoJouvence, 13385 Marseille, France * Correspondence: [email protected] Abstract: Adult stem cells (ASCs) are the undifferentiated cells that possess self-renewal and differ- entiation abilities. They are present in all major organ systems of the body and are uniquely reserved there during development for tissue maintenance during homeostasis, injury, and infection. They do so by promptly modulating the dynamics of proliferation, differentiation, survival, and migration. Any imbalance in these processes may result in regeneration failure or developing cancer. Hence, the dynamics of these various behaviors of ASCs need to always be precisely controlled. Several genetic and epigenetic factors have been demonstrated to be involved in tightly regulating the proliferation, differentiation, and self-renewal of ASCs. Understanding these mechanisms is of great importance, given the role of stem cells in regenerative medicine. Investigations on various animal models have played a significant part in enriching our knowledge and giving In Vivo in-sight into such ASCs regulatory mechanisms. In this review, we have discussed the recent In Vivo studies demonstrating the role of various genetic factors in regulating dynamics of different ASCs viz. -

Tanibirumab (CUI C3490677) Add to Cart
5/17/2018 NCI Metathesaurus Contains Exact Match Begins With Name Code Property Relationship Source ALL Advanced Search NCIm Version: 201706 Version 2.8 (using LexEVS 6.5) Home | NCIt Hierarchy | Sources | Help Suggest changes to this concept Tanibirumab (CUI C3490677) Add to Cart Table of Contents Terms & Properties Synonym Details Relationships By Source Terms & Properties Concept Unique Identifier (CUI): C3490677 NCI Thesaurus Code: C102877 (see NCI Thesaurus info) Semantic Type: Immunologic Factor Semantic Type: Amino Acid, Peptide, or Protein Semantic Type: Pharmacologic Substance NCIt Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor tyrosine kinase expressed by endothelial cells, while VEGF is overexpressed in many tumors and is correlated to tumor progression. PDQ Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor -

Comparison Between Filgrastim and Lenograstim Plus Chemotherapy For
Bone Marrow Transplantation (2010) 45, 277–281 & 2010 Macmillan Publishers Limited All rights reserved 0268-3369/10 $32.00 www.nature.com/bmt ORIGINAL ARTICLE Comparison between filgrastim and lenograstim plus chemotherapy for mobilization of PBPCs R Ria, T Gasparre, G Mangialardi, A Bruno, G Iodice, A Vacca and F Dammacco Department of Biomedical Sciences and Human Oncology, Section of Internal Medicine and Clinical Oncology, University of Bari Medical School, Bari, Italy Recombinant human (rHu) G-CSF has been widely used during transplantation.4 However, the use of G-CSF after to treat neutropenia and mobilize PBPCs for their autologous PBPC transplantation has been queried, as its autologous and allogeneic transplantation. It shortens further reduction in time to a safe neutrophil count5,6 does neutropenia and thus reduces the frequency of neutropenic not always imply fewer significant clinical events, such as fever. We compared the efficiency of glycosylated rHu infections, length of hospitalization, extrahematological and non-glycosylated Hu G-CSF in mobilizing hemato- toxicities or mortality.7,8 Even so, the ASCO guidelines still poietic progenitor cells (HPCs). In total, 86 patients were recommend the use of growth factors after autologous consecutively enrolled for mobilization with CY plus either PBPC transplantation.9 glycosylated or non-glycosylated G-CSF, and under- G-CSF induces the proliferation and differentiation of went leukapheresis. The HPC content of each collection, myeloid precursor cells, and also provides a functional toxicity, days of leukapheresis needed to reach the activity that influences chemotaxis, respiratory burst and minimum HPC target and days to recover WBC (X500 Ag expression of neutrophils. -

G-CSF Protects Motoneurons Against Axotomy-Induced Apoptotic Death In
Henriques et al. BMC Neuroscience 2010, 11:25 http://www.biomedcentral.com/1471-2202/11/25 RESEARCH ARTICLE Open Access G-CSF protects motoneurons against axotomy- induced apoptotic death in neonatal mice Alexandre Henriques1,2,3, Claudia Pitzer1*, Luc Dupuis2,3, Armin Schneider1* Abstract Background: Granulocyte colony stimulating factor (G-CSF) is a growth factor essential for generation of neutrophilic granulocytes. Apart from this hematopoietic function, we have recently uncovered potent neuroprotective and regenerative properties of G-CSF in the central nervous system (CNS). The G-CSF receptor and G-CSF itself are expressed in a motoneurons, G-CSF protects motoneurons, and improves outcome in the SOD1(G93A) transgenic mouse model for amyotrophic lateral sclerosis (ALS). In vitro, G-CSF acts anti- apoptotically on motoneuronal cells. Due to the pleiotrophic effects of G-CSF and the complexity of the SOD1 transgenic ALS models it was however not possible to clearly distinguish between directly mediated anti- apoptotic and indirectly protective effects on motoneurons. Here we studied whether G-CSF is able to protect motoneurons from purely apoptotic cell death induced by a monocausal paradigm, neonatal sciatic nerve axotomy. Results: We performed sciatic nerve axotomy in neonatal mice overexpressing G-CSF in the CNS and found that G-CSF transgenic mice displayed significantly higher numbers of surviving lumbar motoneurons 4 days following axotomy than their littermate controls. Also, surviving motoneurons in G-CSF overexpressing animals were larger, suggesting additional trophic effects of this growth factor. Conclusions: In this model of pure apoptotic cell death the protective effects of G-CSF indicate direct actions of G-CSF on motoneurons in vivo. -

Pharmaceutical Appendix to the Harmonized Tariff Schedule
Harmonized Tariff Schedule of the United States (2019) Revision 13 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2019) Revision 13 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

©Ferrata Storti Foundation
Stem Cell Transplantation • Research Paper Single-dose pegfilgrastim for the mobilization of allogeneic CD34+ peripheral blood progenitor cells in healthy family and unrelated donors Frank Kroschinsky Background and Objectives. Short-term treatment with granulocyte colony-stimulating Kristina Hölig factor (G-CSF) has been established as the standard regimen for mobilizing allogeneic Kirsten Poppe-Thiede peripheral blood progenitor cells (PBPC) from healthy donors. The pegylated form of fil- Kristin Zimmer grastim (pegfilgrastim) has a longer elimination half-life because of decreased serum Rainer Ordemann clearance and might be a convenient alternative for stem cell mobilization. Matthias Blechschmidt Uta Oelschlaegel Design and Methods. Twenty-five family (n=15) or unrelated (n=10) healthy donors received a single-dose of 12 mg pegfilgrastim for mobilization of allogeneic PBPC. Martin Bornhauser + Gabi Rall Donors with inadequate mobilization (blood CD34 cells ≤5/µL on day 3 or ≤20/µL on Claudia Rutt day 4) were given additional daily doses of 10 µg/kg conventional filgrastim. Gerhard Ehninger Leukapheresis was planned to start on day 5. Results. All harvests were completed successfully. In 20 out of 25 donors (80 %) only a single apheresis was necessary. Additional non-pegylated filgrastim had to be given to only one 74-year old family donor. The maximum concentration of circulating CD34+ + cells occurred on day 5 (median 67/µL, range 10-385/µL). The median yield of CD34 cells was 9.3 (range 3.2-39.1) 106/kg of the recipient´s body weight. The median × 8 number of T cells in the apheresis products was 3.9 (range 2.7-10.8)×10 /kg. -

WO 2016/174183 Al 3 November 2016 (03.11.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/174183 Al 3 November 2016 (03.11.2016) P O P C T (51) International Patent Classification: dreas; Pistoriusstr. 7, 13086 Berlin (DE). NEUHAUS, Ro¬ Λ 61Κ 31/4439 (2006.01) A61K 31/519 (2006.01) land; Lauenburger Str. 28, 12157 Berlin (DE). A61K 31/454 (2006.01) A61K 31/5377 (2006.01) (74) Agent: BIP PATENTS; c/o Bayer Intellectual Property A61K 31/496 (2006.01) A61K 31/55 (2006.01) GmbH, Alfred-Nobel-Str. 10, 40789 Monheim am Rhein A61K 31/497 (2006.01) C07D 401/12 (2006.01) (DE). A61K 31/4985 (2006.01) A61P 35/02 (2006.01) A61K 31/505 (2006.01) A61P 35/00 (2006.01) (81) Designated States (unless otherwise indicated, for every A61K 31/506 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/EP2016/059576 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 29 April 2016 (29.04.2016) KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (25) Filing Language: English PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (26) Publication Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Human G-CSF ELISA Kit (ARG80143)
Product datasheet [email protected] ARG80143 Package: 96 wells Human G-CSF ELISA Kit Store at: 4°C Component Cat. No. Component Name Package Temp ARG80143-001 Antibody-coated 8 X 12 strips 4°C. Unused strips microplate should be sealed tightly in the air-tight pouch. ARG80143-002 Standard 3 X 2 ng/vial 4°C (Lyophilized) ARG80143-003 Standard diluent 20 ml 4°C buffer ARG80143-004 Antibody conjugate 400 µl 4°C concentrate ARG80143-005 Antibody diluent 16 ml 4°C buffer ARG80143-006 HRP-Streptavidin 400 µl 4°C (Protect from concentrate light) ARG80143-007 HRP-Streptavidin 16 ml 4°C diluent buffer ARG80143-008 20X Wash buffer 50 ml 4°C ARG80143-009 TMB substrate 12ml 4°C (Protect from light) ARG80143-010 STOP solution 12ml 4°C ARG80143-011 Plate sealer 4 strips Room temperature Summary Product Description ARG80143 Human G-CSF ELISA Kit is an Enzyme Immunoassay kit for the quantification of Human G-CSF in Serum, Plasma, Cell culture supernatants. Tested Reactivity Hu Tested Application ELISA Specificity No significant cross-reactivity or interference with Human CT-1,IL-11,OSM,CNTF,HGF,M-CSF;mouse IL-6;rat IL-6,CNTF Target Name G-CSF Conjugation HRP Conjugation Note Substrate: TMB and read at 450 nm Sensitivity 15 pg/ml Sample Type Serum, Plasma, Cell culture supernatants Standard Range 31.25 - 2000 pg/ml www.arigobio.com 1/3 Sample Volume 100 µl Precision CV: Alternate Names Granulocyte colony-stimulating factor; Lenograstim; C17orf33; GCSF; G-CSF; Filgrastim; Pluripoietin; CSF3OS Application Instructions Assay Time 4 hours Properties Form 96 well Storage instruction Store the kit at 2-8°C. -

STEMGEN® (Ancestim) Product Information Page 1 of 12
STEMGEN® (ancestim) Product Information Page 1 of 12 NAME OF THE DRUG Ancestim is a human stem cell factor (SCF), produced by recombinant DNA technology. STEMGEN® is the Amgen Inc. trademark for ancestim (recombinant methionyl human stem cell factor, r-metHuSCF). DESCRIPTION STEMGEN® is a 166 amino acid protein produced by Escherichia coli (E coli) bacteria into which a gene has been inserted for soluble human stem cell factor. STEMGEN® has a monomeric molecular weight of approximately 18,500 daltons and normally exists as a noncovalently associated dimer. The protein has an amino acid sequence that is identical to the natural sequence predicted from human DNA sequence analysis, except for the addition of an N-terminal methionine retained after expression in E coli. Because STEMGEN® is produced in E coli, the product is nonglycosylated. STEMGEN® is a sterile, white, preservative-free, lyophilised powder for reconstitution and administration as a subcutaneous (SC) injection. Each single-use vial of STEMGEN® contains 1.875 mg of ancestim at a specific activity of 0.84 to 1.8 x 106 U/mg (as measured by a cell mitogenesis assay). Vials of STEMGEN® are reconstituted with 1.2 mL of sterile Water for Injection to yield an ancestim concentration of 1500 µg/mL. Reconstituted STEMGEN® is a sterile aqueous solution containing 4.5% mannitol and 0.5% sucrose buffered at pH 6.0 with 5 mM glutamic acid and 10 mM histidine. PHARMACOLOGY Pharmacokinetics General The pharmacokinetics of STEMGEN® are dose linear in the range of 5 to 30 µg/kg in both healthy volunteers and cancer patients. -
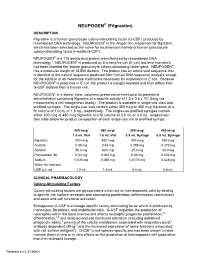
NEUPOGEN® (Filgrastim) DESCRIPTION Filgrastim Is a Human Granulocyte Colony-Stimulating Factor (G-CSF)‚ Produced by Recombinant DNA Technology
NEUPOGEN® (Filgrastim) DESCRIPTION Filgrastim is a human granulocyte colony-stimulating factor (G-CSF)‚ produced by recombinant DNA technology. NEUPOGEN® is the Amgen Inc. trademark for filgrastim‚ which has been selected as the name for recombinant methionyl human granulocyte colony-stimulating factor (r-metHuG-CSF). NEUPOGEN® is a 175 amino acid protein manufactured by recombinant DNA technology.1 NEUPOGEN® is produced by Escherichia coli (E coli) bacteria into which has been inserted the human granulocyte colony-stimulating factor gene. NEUPOGEN® has a molecular weight of 18‚800 daltons. The protein has an amino acid sequence that is identical to the natural sequence predicted from human DNA sequence analysis‚ except for the addition of an N-terminal methionine necessary for expression in E coli. Because NEUPOGEN® is produced in E coli‚ the product is nonglycosylated and thus differs from G-CSF isolated from a human cell. NEUPOGEN is a sterile‚ clear‚ colorless‚ preservative-free liquid for parenteral administration containing filgrastim at a specific activity of 1.0 ± 0.6 x 108 U/mg (as measured by a cell mitogenesis assay). The product is available in single-use vials and prefilled syringes. The single-use vials contain either 300 mcg or 480 mcg filgrastim at a fill volume of 1.0 mL or 1.6 mL, respectively. The single-use prefilled syringes contain either 300 mcg or 480 mcg filgrastim at a fill volume of 0.5 mL or 0.8 mL, respectively. See table below for product composition of each single-use vial or prefilled syringe. 300 mcg/ 480 mcg/ 300 mcg/ 480 mcg/ 1.0 mL Vial 1.6 mL Vial 0.5 mL Syringe 0.8 mL Syringe filgrastim 300 mcg 480 mcg 300 mcg 480 mcg Acetate 0.59 mg 0.94 mg 0.295 mg 0.472 mg Sorbitol 50.0 mg 80.0 mg 25.0 mg 40.0 mg Polysorbate 80 0.04 mg 0.064 mg 0.02 mg 0.032 mg Sodium 0.035 mg 0.056 mg 0.0175 mg 0.028 mg Water for Injection USP q.s.