Diagenesis of Upper Cambrian Mount Simon Sandstone in the Illinois Basin - Microscale Investigation of Basinal Fluid Migration and Mass Transfer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1920 503800 Passenger Vehicles Are
1920 503,800 passenger vehicles are registered with the Illinois Secre- tary of State’s office. Trucks begin to be registered separately from passenger cars. The prefix TRUCK appears vertically on these license plates. Truck owners are required to display a metal truck plate, attached to the license plate, with the word “ILLINOIS WEIGHT” and the maximum weight of the vehicle and the load permitted embossed on it. The plate must be attached to the vehicle at all times in a conspicuous place. Greenduck Co. of Chicago is contracted to make the plates. However, 100,000 passenger plates and the metal truck plates are sublet to Grimm Stamp and Badge Co. of St. Louis. 1921 583,400 passenger vehicles are registered with the Illinois Secre- tary of State’s office. License plates are made by four different companies: • Grimm Stamp and Badge Co. of St. Louis, MO, numbers 1 to 200-000 • Western Display Co. of St. Paul, MN, numbers 200-001 to 300-000 • C.H. Hanson Co. of Chicago, numbers 300-001 to 500-000 • Greenduck Co. of Chicago, non-passenger plates. The first highway police force is created on June 24 when the 1 Illinois General Assembly authorizes the Illinois Department of Public Works and Buildings to appoint a sufficient number of State Highway Maintenance Police to patrol the highways and make arrests for violations of the Motor Vehicle Law. 1922 682,300 passenger vehicles are registered with the Illinois Secre- tary of State’s office. The increase in the number of automobiles necessitates more policing. In 1922, the Secretary of State’s office is granted police powers for the enforcement of automobile laws. -

Guidelines for Recreation and Park Systems
DOCUMENT RESUME ED 057 968 RC 005 858 AUTHOR Bannon, Joseph J.; Storey, Edward H. TITLE Guidelines for Recreation and Park Systems INSTITUTION Illinois Univ., Urbana. SPONS AGENCY Department of Agriculture, Washington, D.C. REPORT NO Circ-1017 PUB DATE Jun 70 NOTE 26p. EDFS PRIcE MF-$0.65 HC-$3.29 DESCRIPTORS *City Planning; rommun ty Involvement; *Educational Facilities; Government Role; Individual Needs: *Land Use; Needs; Park Design; *narks; *Recreational Facilities; Regional Planning; Shared Services; State Laws ABSTRACT In this publication, written for use in guiding community recreation and park systems, the following topics are discussed: why parks and recreational facilities should be developed, the need for governmental participation, and park-system development. Additionally, neighborhood parks, playlots, community parks, city-wide parks, regional parks and reservations, and specialized recreation areas and facilities are examined in terms of desirable physical characteristics and user populations. Principles applying to design of park and recreation areas and cooperative facility usafe by school and community are also reviewed. To provide examples of joint development of school-recreation facilities, 4 communities are described: Topeka, Kansas; Mount Prospect, Illinois; Austin, Texas; and Elk Grove Village, Illinois. An outline of recommended community park and recreation practices discusses administration, community cooperation, planning, facilities, programs, and personnel, and a chart of selected Illinois laws is included. (MJB) Contents Why Develop Parks and Recreation Facilities9 2 The Need for Government Participation 4 Guidelines for Park System Development 6 Distribution in the Park System ......... .. .. 7 Neighborhood Parks Playlots 8 Community Parks 9 City-Wide Parks Regional Parks and Reservations....... .... 10 Specialized Recreation Areas and Facilities 11 Designini Parks and Recreation Areas 11 Using School F :militias in Park and Recreation Systems 12 Examples of Joint Development ...... -

Cambrian Ordovician
Open File Report LXXVI the shale is also variously colored. Glauconite is generally abundant in the formation. The Eau Claire A Summary of the Stratigraphy of the increases in thickness southward in the Southern Peninsula of Michigan where it becomes much more Southern Peninsula of Michigan * dolomitic. by: The Dresbach sandstone is a fine to medium grained E. J. Baltrusaites, C. K. Clark, G. V. Cohee, R. P. Grant sandstone with well rounded and angular quartz grains. W. A. Kelly, K. K. Landes, G. D. Lindberg and R. B. Thin beds of argillaceous dolomite may occur locally in Newcombe of the Michigan Geological Society * the sandstone. It is about 100 feet thick in the Southern Peninsula of Michigan but is absent in Northern Indiana. The Franconia sandstone is a fine to medium grained Cambrian glauconitic and dolomitic sandstone. It is from 10 to 20 Cambrian rocks in the Southern Peninsula of Michigan feet thick where present in the Southern Peninsula. consist of sandstone, dolomite, and some shale. These * See last page rocks, Lake Superior sandstone, which are of Upper Cambrian age overlie pre-Cambrian rocks and are The Trempealeau is predominantly a buff to light brown divided into the Jacobsville sandstone overlain by the dolomite with a minor amount of sandy, glauconitic Munising. The Munising sandstone at the north is dolomite and dolomitic shale in the basal part. Zones of divided southward into the following formations in sandy dolomite are in the Trempealeau in addition to the ascending order: Mount Simon, Eau Claire, Dresbach basal part. A small amount of chert may be found in and Franconia sandstones overlain by the Trampealeau various places in the formation. -

Long-Range Petroleum Migration in the Illinois Basin CRAIG M
ILLINOIS STATE GEOLOGICAL SURVEY Interior Cratonic Basins, 1991, edited by Reprint Series 1991 - T26 M. W. Leighton, D. R. Kalata, D. F. Oltz, and J. J. Eidel: American Association of Petroleum Geologists Memoir 51,819 p. CHAPTER 26 Long-Range Petroleum Migration in the Illinois Basin CRAIG M. BETHKE DONALD F. OLTZ University of Illinois Illinois State Geological Survey Urbana, Illinois Champaign, Illinois JACKIE D. REED ARCO Oil and Gas Company Plano, Texas Editor's note: No treatment of oil and gas in the Illinois In this section, we define the migration that has basin would be complete without an overview of occurred in the Illinois basin on the basis of shale hydrocarbon migration. The following is a condensed petrographic observations and geochemical correla version, made especially for this volume, of an article tions. We emphasize the origin of oils found in shallow that will appear in the American Association of reservoirs, mostly in Silurian strata to the north and Petroleum Geologists Bulletin in 1991. northwest of the central basin (Figure 26-2), because these oils seem to have migrated farthest from source beds. We show that these oils, which are produced from reservoirs more than 125 mi (200 km) from the basin's depocenter, were derived from Devonian source rocks in the deep basin. We then use quantitative models of the basin's paleohydrology to INTRODUCTION investigate the past subsurface conditions that drove the migration. We conclude that long-range migration The distribution of petroleum production from the was not related to processes occurring as the basin Illinois basin extends considerably beyond the area subsided and infilled. -

Geology of the Devonian Marcellus Shale—Valley and Ridge Province
Geology of the Devonian Marcellus Shale—Valley and Ridge Province, Virginia and West Virginia— A Field Trip Guidebook for the American Association of Petroleum Geologists Eastern Section Meeting, September 28–29, 2011 Open-File Report 2012–1194 U.S. Department of the Interior U.S. Geological Survey Geology of the Devonian Marcellus Shale—Valley and Ridge Province, Virginia and West Virginia— A Field Trip Guidebook for the American Association of Petroleum Geologists Eastern Section Meeting, September 28–29, 2011 By Catherine B. Enomoto1, James L. Coleman, Jr.1, John T. Haynes2, Steven J. Whitmeyer2, Ronald R. McDowell3, J. Eric Lewis3, Tyler P. Spear3, and Christopher S. Swezey1 1U.S. Geological Survey, Reston, VA 20192 2 James Madison University, Harrisonburg, VA 22807 3 West Virginia Geological and Economic Survey, Morgantown, WV 26508 Open-File Report 2012–1194 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior Ken Salazar, Secretary U.S. Geological Survey Marcia K. McNutt, Director U.S. Geological Survey, Reston, Virginia: 2012 For product and ordering information: World Wide Web: http://www.usgs.gov/pubprod Telephone: 1-888-ASK-USGS For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment: World Wide Web: http://www.usgs.gov Telephone: 1-888-ASK-USGS Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted material contained within this report. -

APRIL A. OTTERBERG Partner APRIL A. OTTERBERG
APRIL A. OTTERBERG, Partner April A. Otterberg concentrates on professional liability litigation, with more than a decade of experience litigating complex professional liability disputes. She defends lawyers and law firms in pending or threatened legal malpractice and related claims, including aiding and abetting claims, claims from non-clients, and claims brought by receivers, and she defends other types of professionals targeted for alleged errors or defects in the services they provide. Ms. Otterberg has substantial experience investigating professional liability claims and developing and mounting effective and practical defense strategies, whether pre-complaint or through the litigation process. She also represents corporate clients in breach of fiduciary duty and related claims under ERISA, and she has experience representing corporations and employees in trade secret and restrictive covenant cases. APRIL A. OTTERBERG Ms. Otterberg has represented clients in a range of civil litigation matters, Partner including putative class actions, appellate matters, cases litigated successfully CHICAGO at trial, and several matters involving pre-suit counseling, mediation, and Office: 312 840-8646 settlement negotiations. She is a member of the firm’s Professional Fax: 312 840-8746 Responsibility, ERISA Litigation, and Trade Secret and Restrictive Covenant Email: [email protected] Litigation and Counseling practices. PRACTICE GROUPS Ms. Otterberg has focused her pro bono efforts on criminal defense matters, Energy ERISA Litigation including her longtime representation of a client on federal death row in Intellectual Property connection with his appeal and post-conviction proceedings. She is co-author of Litigation the Illinois portion of the American Bar Association’s The Law of Lawyers’ Professional Responsibility Liability and of the firm’s outline of Illinois trade secrets law. -

Geologic and Geochemical Studies of the New Albany Shale Group (Devonian-Mississippian) in Illinois
557 3M SuMr IL6of 1980-2 Final Report Geologic and Geochemical Studies of the New Albany Shale Group (Devonian-Mississippian) in Illinois R. E. Bergstrom and N. F. ShimP/ Principal Invistigators Robert M. Cluff, Project Coordinator Illinois State Geological Survey Open File Series 1980-2 June 30, 1980 Illinois State Geological Survey Urbana, IL 61801 Prepared for U.S. Department of Energy Morgantown Energy Technology Center Morgantown, WV 2 6505 Contract: U.S. Department of Energy DE-AC21-76ET12142 , Note to readers: Printouts of the data contained on the computer file and copies of the structure map mentioned in this report can be obtained from the Illinois State Geological Survey upon written request, This report was prepared as an account of work partially sponsored by the U.S. Government. Neither the United States nor the United States Depart- ment of Energy, nor any of their employees , contractors, subcontractors or employees makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. '^'NOIS STATE QEOLOQICAL SURVEY 3 305 Final Report Geologic and Geochemical Studies of the New Albany Shale Group (Devonian-Mississippian) in Illinois R. E. Bergstrom and N. F. Shimp, Principal Invistigators Robert M. Cluff, Project Coordinator Illinois State Geological Survey Open File Series 1980-2 SEP 1 1 1996 |L uluu ourWtY June 30, 1980 Illinois State Geological Survey Urbana, IL 61801 Prepared for U.S. Department of Energy Morgantown Energy Technology Center Morgantown, WV 2 6505 Contract: U.S. -

Governors Conference on the Management of the Illinois River
Proceedings 1995 Governor's Conference On the Management of the Illinois River System Fifth Biennial Conference October 10-11, 1995 Hotel P_re Marquette Peoria, Illinois Proceedings 1995 Governor's Conference On the Management of the Illinois River System Fifth Biennial Conference October 10-11, 1995 Hotel P_re Marquette Peoria, Illinois Elizabeth D. Wagner, Editor Institute for Environmental Studies Issued as Special Report No. 22 Water Resources Center Institute for Environmental Studies University of minois at Urbana-Champaign 1101 W. Peabody Dr., Urbana, IL 61801 (217) 333-0536 Photographsby Jon Hubbert, Natural Resources Conservation Service, Department of Agriculture. Printed with financial support from the Illinois Department of Natural Resources. (450/February 1996) Printed on recycled paper. Contents Acknowledgments Opening Address Robert W. Frazee .............................................. 3 Keynote Speech Sharing the Challenge: Floodplain Management Into the 21st Century Brigadier General Gerald E. Galloway, Jr .............................. 7 Session L The Past Navigation on the Illinois Donald R. Vonnahme and Bruce Barker ............................. 19 Illinois Possesses Unique Knowledge About Its Weather and Climate Stanley A. Changnon ........................................... 29 A Century of Water Resources Research at the Illinois State Water Survey: Meeting the Challenge Mike Demissie, Vernon Knapp and Adrian Visocky ...................... 43 Forbes Biological Station Commemorates 100 Years of Research, S.P. Havera and K.E. Roat ....................................... 53 Keynote Speech Economic/mpact of the _linois River on River Communities David R. Allardice .. ...... 59 Session IL The Present An "NRI Snapshot" of Resource Conditions in the Illinois River Drainage Basin Robert McLeese .............................................. 75 nlinffts T by 2000 Transect Survey, 1995 Results Chet Boruff ................................................. 83 No-Till in the lllinois River Watershed Robert W. -

Mineral Resources of the Illinois Basin in the Context of Basin Evolution
Mineral Resources of the Illinois Basin in the Context of Basin Evolution St. Louis, Missouri, January 22-23,1992 Program and Abstracts Edited by Martin B. Goldhaber and J. James Eidel U.S. Geological Survey Open-File Report 92-1 This report is preliminary and has not been edited or reviewed for conformity with U.S. Geological Survey, Illinois State Geological Survey, Kentucky Geological Survey, Missouri Division of Geology and Land Survey, and Indiana Geological Survey standards. PREFACE The mineral resources of the U.S. midcontinent were instrumental to the development of the U.S. economy. Mineral resources are an important and essential component of the current economy and will continue to play a vital role in the future. Mineral resources provide essential raw materials for the goods consumed by industry and the public. To assess the availability of mineral resources and contribute to the abib'ty to locate and define mineral resources, the U.S. Geological Survey (USGS) has undertaken two programs in cooperation with the State Geological Surveys in the midcontinent region. In 1975, under the Conterminous U.S. Mineral Assessment Program (CUSMAP) work began on the Rolla 1° X 2° Quadrangle at a scale of 1:250,000 and was continued in the adjacent Springfield, Harrison, Joplin, and Paducah quadrangles across southern Missouri, Kansas, Illinois, Arkansas, and Oklahoma. Public meetings were held in 1981 to present results from the Rolla CUSMAP and in 1985 for the Springfield CUSMAP. In 1984, the Midcontinent Strategic and Critical Minerals Project (SCMP) was initiated by the USGS and the State Geological Surveys of 16 states to map and compile data at 1:1,000,000 scale and conduct related topical studies for the area from latitude 36° to 46° N. -

Reservoir Quality and Diagenetic
U.S. Department of the Interior U.S. Geological Survey Reservoir Quality and Diagenetic Evolution of Upper Mississippian Rocks in the Illinois Basin: Influence of a Regional Hydrothermal Fluid-Flow Event During Late Diagenesis U.S. Geological Survey Professional Paper 1597 Availability of Publications of the U.S. Geological Survey Order U.S. Geological Survey (USGS) publications from the Documents. Check or money order must be payable to the offices listed below. Detailed ordering instructions, along with Superintendent of Documents. Order by mail from— prices of the last offerings, are given in the current-year issues of the catalog "New Publications of the U.S. Geological Superintendent of Documents Survey." Government Printing Office Washington, DC 20402 Books, Maps, and Other Publications Information Periodicals By Mail Many Information Periodicals products are available through Books, maps, and other publications are available by mail the systems or formats listed below: from— Printed Products USGS Information Services Box 25286, Federal Center Printed copies of the Minerals Yearbook and the Mineral Com Denver, CO 80225 modity Summaries can be ordered from the Superintendent of Publications include Professional Papers, Bulletins, Water- Documents, Government Printing Office (address above). Supply Papers, Techniques of Water-Resources Investigations, Printed copies of Metal Industry Indicators and Mineral Indus Circulars, Fact Sheets, publications of general interest, single try Surveys can be ordered from the Center for Disease Control copies of permanent USGS catalogs, and topographic and and Prevention, National Institute for Occupational Safety and thematic maps. Health, Pittsburgh Research Center, P.O. Box 18070, Pitts burgh, PA 15236-0070. Over the Counter Mines FaxBack: Return fax service Books, maps, and other publications of the U.S. -
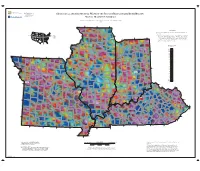
Geological and Geophysical Maps of the Illinois Basin-Ozark Dome Region
Prairie Research Institute Illinois State Geological Survey 615 East Peabody Drive GEOLOGICAL AND GEOPHYSICAL MAPS OF THE ILLINOIS BASIN–OZARK DOME REGION Champaign, Illinois 61820-6918 (217) 244-2414 http://www.isgs.illinois.edu MAP 10: MAGNETIC ANOMALY Digitally compiled by Curtis C. Abert, Stephen Marshak, and Timothy H. Larson 2016 90° 89° 88° Data Source 90° 0' 0" W Raster cell size is approximately 1,026 meters. Data were extracted from the fol- lowing source: 40° 0' 0" N STATES Bankey, V., A. Cuevas, D. Daniels, C.A. Finn, I. Hernandez, P. Hill, R. Kucks, W. 1 Missouri 42° 42° Miles, M. Pilkington, C. Roberts, W. Roest, V. Rystrom, S. Shearer, S. Snyder, 2 3 2 Illinois 3 Indiana 85° R. Sweeney, J. Velez, J.D. Phillips, and D. Ravat, 2002, Digital data grids for 1 4 86° 4 Kentucky Chicago! the magnetic anomaly map of North America: Denver, Colorado, U.S. Geo- 87° logical Survey, Open-File Report 02-414, http://mrdata.usgs.gov/geophysics/ aeromag.html (accessed September 9, 2015). 91° Magnetic Anomaly Nanotesla (nT) 400 200 41° 150 41° 100 90 95° 94° 70 93° 92° 50 30 20 10 0 40° Ϫ10 40° Ϫ20 Ϫ30 Ϫ40 ! Indianapolis Ϫ60 Ϫ80 Ϫ100 Ϫ125 95° Ϫ175 Ϫ200 Ϫ300 ! Kansas City 39° 39° 84° 83° St. Louis! Louisville ! 38° 38° 82° 82° 37° 37° 83° 85° 84° 87° 86° 94° 93° 92° 91° 89° 88° 36° 36° 90° 1: 1,500,000 Vector base data from 2009 Esri Data and Maps Digital cartography by Curtis C. Abert and Jennifer E. -

Mississippian Oolites and Petroleum Reservoirs in the United States—An Overview
Chapter 1 • Mississippian Oolites and Petroleum Reservoirs in the United States—An Overview Brian D. Keith Indiana Geological Survey and Indiana University Bloomington, Indiana, USA Charles W. Zuppann Indiana Geological Survey Bloomington, Indiana, USA • ABSTRACT A coincidence of tectonic, eustatic, and geochemical conditions resulted in substantial deposits of oolitic limestone during later Mississippian time in the continental United States. These oolitic limestones have formed petrole um reservoirs with favorable primary and secondary recovery characteris tics. Significant potential reserves in stratigraphic traps remain to be discovered and developed in these reservoirs. INTRODUCTION oolitic limestone deposition on the North American continent was during the Mississippian. Mississippian rocks of the continental U.S. have With the recent emphasis on global-scale geologic been the subject of two previous compilation vol processes, we recognize that attention must be given umes by the USGS (Craig and Connor, 1979; U.S. to Mississippian oolitic deposition on a broader scale, Geological Survey, 1979), both concentrating on the seeking to understand the factors that influenced and Mississippian system as a whole, the former on controlled oolitic deposition at that time. A prerequi Mississippian and Pennsylvanian stratigraphy and site to interpreting worldwide depositional patterns the latter on the paleotectonic history of the of Mississippian oolitic rocks is to study these Mississippian. However, there has not been a publi deposits at both the regional and the continental cation specifically emphasizing Mississippian oolitic scales. The abundant occurrence and widespread dis rocks. Geologists have reported oolitic limestones in tribution of Mississippian oolitic rocks, and the Mississippian rocks in many areas of the United wealth of available subsurface information, make the States for years, but Wilson (1975, p.