Wsn 74 (2017) 80-93 Eissn 2392-2192
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nigeria's Constitution of 1999
PDF generated: 26 Aug 2021, 16:42 constituteproject.org Nigeria's Constitution of 1999 This complete constitution has been generated from excerpts of texts from the repository of the Comparative Constitutions Project, and distributed on constituteproject.org. constituteproject.org PDF generated: 26 Aug 2021, 16:42 Table of contents Preamble . 5 Chapter I: General Provisions . 5 Part I: Federal Republic of Nigeria . 5 Part II: Powers of the Federal Republic of Nigeria . 6 Chapter II: Fundamental Objectives and Directive Principles of State Policy . 13 Chapter III: Citizenship . 17 Chapter IV: Fundamental Rights . 20 Chapter V: The Legislature . 28 Part I: National Assembly . 28 A. Composition and Staff of National Assembly . 28 B. Procedure for Summoning and Dissolution of National Assembly . 29 C. Qualifications for Membership of National Assembly and Right of Attendance . 32 D. Elections to National Assembly . 35 E. Powers and Control over Public Funds . 36 Part II: House of Assembly of a State . 40 A. Composition and Staff of House of Assembly . 40 B. Procedure for Summoning and Dissolution of House of Assembly . 41 C. Qualification for Membership of House of Assembly and Right of Attendance . 43 D. Elections to a House of Assembly . 45 E. Powers and Control over Public Funds . 47 Chapter VI: The Executive . 50 Part I: Federal Executive . 50 A. The President of the Federation . 50 B. Establishment of Certain Federal Executive Bodies . 58 C. Public Revenue . 61 D. The Public Service of the Federation . 63 Part II: State Executive . 65 A. Governor of a State . 65 B. Establishment of Certain State Executive Bodies . -

Prof. Dr. Kayode AJAYI Dr. Muyiwa ADEYEMI Faculty of Education Olabisi Onabanjo University, Ago-Iwoye, NIGERIA
International Journal on New Trends in Education and Their Implications April, May, June 2011 Volume: 2 Issue: 2 Article: 4 ISSN 1309-6249 UNIVERSAL BASIC EDUCATION (UBE) POLICY IMPLEMENTATION IN FACILITIES PROVISION: Ogun State as a Case Study Prof. Dr. Kayode AJAYI Dr. Muyiwa ADEYEMI Faculty of Education Olabisi Onabanjo University, Ago-Iwoye, NIGERIA ABSTRACT The Universal Basic Education Programme (UBE) which encompasses primary and junior secondary education for all children (covering the first nine years of schooling), nomadic education and literacy and non-formal education in Nigeria have adopted the “collaborative/partnership approach”. In Ogun State, the UBE Act was passed into law in 2005 after that of the Federal government in 2004, hence, the demonstration of the intention to make the UBE free, compulsory and universal. The aspects of the policy which is capital intensive require the government to provide adequately for basic education in the area of organization, funding, staff development, facilities, among others. With the commencement of the scheme in 1999/2000 until-date, Ogun State, especially in the area of facility provision, has joined in the collaborative effort with the Federal government through counter-part funding to provide some facilities to schools in the State, especially at the Primary level. These facilities include textbooks (in core subjects’ areas- Mathematics, English, Social Studies and Primary Science), blocks of classrooms, furniture, laboratories/library, teachers, etc. This study attempts to assess the level of articulation by the Ogun State Government of its UBE policy within the general framework of the scheme in providing facilities to schools at the primary level. -

Prevalence, Intensity and Spatial Co-Distribution of Schistosomiasis
Parasitology Open Prevalence, intensity and spatial co-distribution of schistosomiasis and cambridge.org/pao soil transmitted helminths infections in Ogun state, Nigeria Research Article 1 1 1,2 Cite this article: Oluwole AS, Adeniran AA, Akinola S. Oluwole , Adebiyi A. Adeniran , Hammed O. Mogaji , Mogaji HO, Olabinke DB, Abe EM, Bankole SO, Dorcas B. Olabinke1, Eniola M. Abe1,3, Samuel O. Bankole1, Sam-Wobo SO, Ekpo UF (2018). Prevalence, intensity and spatial co-distribution of Sammy O. Sam-Wobo1 and Uwem F. Ekpo1 schistosomiasis and soil transmitted helminths infections in Ogun state, Nigeria. 1Department of Pure and Applied Zoology, Federal University of Agriculture, Abeokuta, Nigeria; 2Department of Parasitology Open 4, e8, 1–9. https://doi.org/ Zoology, Federal University, Oye-Ekiti, Nigeria and 3National Institute of Parasitic Diseases, Chinese Centre for 10.1017/pao.2018.4 Disease Prevention, WHO Collaborating Centre for Tropical Diseases, Shanghai, China Received: 26 March 2017 Revised: 9 February 2018 Abstract Accepted: 9 February 2018 A cross-sectional survey was carried out in primary schools to determine prevalence, intensity Key words: and spatial co-distribution of Schistosomiasis and soil transmitted helminths (STH) infections Co-distribution; prevalence; schistosomiasis; in Ogun State, Nigeria. A total of 2148 pupils from 42 schools were examined for Schistosoma soil transmitted helminths; spatial risk and STH infections from urine and fresh fecal samples respectively. Ethyl ether concentration Author for correspondence: method prepared in sodium acetate – acetic acid – formalin ether was used to concentrate Akinola S. Oluwole, E-mail: akinolaoluwole@ parasites’ ova before microscopic examination. The overall prevalence of schistosomiasis gmail.com and STH infections were 4.0% (95% CI = 3.21–4.92) and 34.64% (95% CI = 32.62–36.69) respectively. -
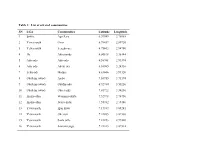
Table 1: List of Selected Communities SN LGA Communities Latitude
Table 1: List of selected communities SN LGA Communities Latitude Longitude 1 Ipokia Ago Sasa 6.59089 2.76065 2 Yewa-south Owo 6.78457 2.89720 3 Yewa-south Ireagbo-are 6.75602 2.94780 4 Ifo Akinsinnde 6.80818 3.16144 5 Ado-odo Ado-odo 6.58768 2.93374 6 Ado-odo Abebi-ota 6.68965 3.24330 7 Ijebu-ode Molipa 6.83606 3.91120 8 Obafemi-owode Ajebo 7.10955 3.71174 9 Obafemi-owode Odofin-odo 6.92744 3.55220 10 Obafemi-owode Oba-seriki 7.01712 3.34230 11 Imeko-afon Wasinmi-okuta 7.52948 2.76750 12 Imeko-afon Iwoye-ketu 7.55782 2.74486 13 Yewa-north Igan ikoto 7.15339 3.04281 14 Yewa-north Oke rori 7.24805 3.02368 15 Yewa-north Saala orile 7.21253 2.97420 16 Yewa-north Araromi joga 7.23323 3.02514 17 Ewekoro Abule Oko 6.86859 3.19430 18 Shagamu Ipoji 6.84440 3.65006 19 Shagamu Odelemo 6.74479 3.66392 20 Ikenne Irolu 6.90834 3.72447 21 Odogbolu Ikosa 6.83873 3.76291 22 Ijebu-east Itele 6.76299 4.06629 23 Ijebu-east Imobi 6.65920 4.17934 24 Ijebu north-east Atan 6.89712 4.00414 25 Abeokuta-south Ibon 7.15864 3.35519 26 Ijebu north Agric 6.93907 3.83253 27 Ijebu north Japara 6.97274 3.99278 28 Remo north Akaka 6.94053 3.71328 29 Odeda Alabata 7.31567 3.53351 30 Odeda Olodo 7.29659 3.60758 31 Abeokuta north Imala odo 7.32122 3.18115 32 Ogun water-side Abigi 6.48618 4.39408 33 Ogun water-side Iwopin 6.51054 4.16990 Table 2: Sex and age distribution of study participants SN LGA Sex (%) Age in years (%) Number Male Female <5yrs 5-15yrs 16-25yrs 26-40yrs 41-70yrs >70yrs Examined 1 Abeokuta north 87 28(32.2) 59(67.8) 7(8.0) 64(7.6) 9(10.3) 3(3.4) 4(4.6) -

Spatial Distribution of Ascariasis, Trichuriasis and Hookworm Infections in Ogun State, Southwestern Nigeria
Spatial Distribution of Ascariasis, Trichuriasis and Hookworm Infections in Ogun State, Southwestern Nigeria Hammed Mogaji ( [email protected] ) Federal University Oye-Ekiti https://orcid.org/0000-0001-7330-2892 Gabriel Adewunmi Dedeke Federal University of Agriculture Abeokuta Babatunde Saheed Bada Federal University of Agriculture Abeokuta Samuel O. Bankole Federal University of Agriculture Abeokuta Adejuwon Adeniji Federal University of Agriculture Abeokuta Mariam Tobi Fagbenro Federal University of Agriculture Abeokuta Olaitan Olamide Omitola Federal University of Agriculture Abeokuta Akinola Stephen Oluwole SightSavers Nnayere Simon Odoemene Adeleke University Uwem Friday Ekpo Federal University of Agriculture Abeokuta Research article Keywords: Spatial Mapping, Distribution, Ascariasis, Trichuriasis, Hookworm, Ogun State, Nigeria Posted Date: July 29th, 2019 DOI: https://doi.org/10.21203/rs.2.12035/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/20 Abstract Background Ascariasis, Trichuriasis and Hookworm infections poses a considerable public health burden in Sub-Saharan Africa, and a sound understanding of their spatial distribution facilitates to better target control interventions. This study, therefore, assessed the prevalence of the trio, and mapped their spatial distribution in the 20 administrative regions of Ogun State, Nigeria. Methods Parasitological surveys were carried out in 1,499 households across 33 spatially selected communities. Fresh stool samples were collected from 1,027 consenting participants and processed using ether concentration method. Households were georeferenced using a GPS device while demographic data were obtained using a standardized form. Data were analysed using SPSS software and visualizations and plotting maps were made in ArcGIS software. Results Findings showed that 19 of the 20 regions were endemic for one or more kind of the three infections, with an aggregated prevalence of 17.2%. -
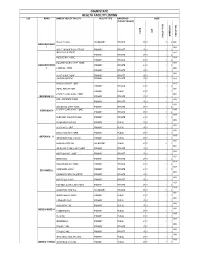
List of Coded Health Facilities in Ogun State.Pdf
OGUN STATE HEALTH FACILITY LISTING LGA WARD NAME OF HEALTH FACILITY FACILITY TYPE OWNERSHIP CODE (PUBLIC/ PRIVATE) LGA STATE OWNERSHIP FACILITY NO FACILITY FACILITY TYPE FACILITY Shallom Hospital SECONDARY PRIVATE 27 01 2 2 0001 IBEREKODO WARD 1 0002 UNCLE JOHN MEDICAL SERVICE PRIMARY PRIVATE 27 01 1 2 INSHA ALAHU EWEJE 0003 PRIMARY PRIVATE 27 01 1 2 MODUPE MAT. HOME 0004 PRIMARY PRIVATE 27 01 1 2 COLUMBIA CLINIC & MAT. HOME 0005 IBEREKODO WARD PRIMARY PRIVATE 27 01 1 2 2 LISABI MAT. HOME 0006 PRIMARY PRIVATE 27 01 1 2 0007 ALASELA MAT. HOME PRIMARY PRIVATE 27 01 1 2 JULIBAM HOSPITAL PRIMARY PRIVATE 27 01 1 2 0008 KING DAVIDS MAT. HOME 0009 PRIMARY PRIVATE 27 01 1 2 ROYAL HEALTH POST 0010 PRIMARY PUBLIC 27 01 1 1 CHARITY CLINIC & MAT. HOME 0011 IBEREKODO 111 PRIMARY PRIVATE 27 01 1 2 ADE – FAITH MAT. HOME 0012 PRIMARY PRIVATE 27 01 1 2 0013 GOD GRACE & MAT. HOME PRIMARY PRIVATE 27 01 1 2 IBEREKODO IV CHARITY CLINIC & MAT. HOME 0014 PRIMARY PRIVATE 27 01 1 2 0015 DURO MED. DIAGNOSTE LAB PRIMARY PRIVATE 27 01 1 2 0016 ELEGA HEALTH CLINIC PRIMARY PUBLIC 27 01 1 1 0017 ALAFIA MAT. HOME PRIMARY PUBLIC 27 01 1 1 0018 OMOLAYAJO MAT. HOME PRIMARY PUBLIC 27 01 1 1 IBEREKODO V 0019 IBEREKODO HEALTH CLINIC PRIMARY PUBLIC 27 01 1 1 0020 GENERAL HOSPITAL SECONDARY PUBLIC 27 01 2 1 0021 IKE-OLUWA CLINIC & MAT. HOME PRIMARY PRIVATE 27 01 1 2 0022 MOPELOLA MAT. HOME PRIMARY PRIVATE 27 01 1 2 0023 BISTED MED PRIMARY PRIVATE 27 01 1 2 0024 TOLUWALASE MAT HOME PRIMARY PRIVATE 27 01 1 2 0025 LADE MAMO. -

States and Lcdas Codes.Cdr
PFA CODES 28 UKANEFUN KPK AK 6 CHIBOK CBK BO 8 ETSAKO-EAST AGD ED 20 ONUIMO KWE IM 32 RIMIN-GADO RMG KN KWARA 9 IJEBU-NORTH JGB OG 30 OYO-EAST YYY OY YOBE 1 Stanbic IBTC Pension Managers Limited 0021 29 URU OFFONG ORUKO UFG AK 7 DAMBOA DAM BO 9 ETSAKO-WEST AUC ED 21 ORLU RLU IM 33 ROGO RGG KN S/N LGA NAME LGA STATE 10 IJEBU-NORTH-EAST JNE OG 31 SAKI-EAST GMD OY S/N LGA NAME LGA STATE 2 Premium Pension Limited 0022 30 URUAN DUU AK 8 DIKWA DKW BO 10 IGUEBEN GUE ED 22 ORSU AWT IM 34 SHANONO SNN KN CODE CODE 11 IJEBU-ODE JBD OG 32 SAKI-WEST SHK OY CODE CODE 3 Leadway Pensure PFA Limited 0023 31 UYO UYY AK 9 GUBIO GUB BO 11 IKPOBA-OKHA DGE ED 23 ORU-EAST MMA IM 35 SUMAILA SML KN 1 ASA AFN KW 12 IKENNE KNN OG 33 SURULERE RSD OY 1 BADE GSH YB 4 Sigma Pensions Limited 0024 10 GUZAMALA GZM BO 12 OREDO BEN ED 24 ORU-WEST NGB IM 36 TAKAI TAK KN 2 BARUTEN KSB KW 13 IMEKO-AFON MEK OG 2 BOSARI DPH YB 5 Pensions Alliance Limited 0025 ANAMBRA 11 GWOZA GZA BO 13 ORHIONMWON ABD ED 25 OWERRI-MUNICIPAL WER IM 37 TARAUNI TRN KN 3 EDU LAF KW 14 IPOKIA PKA OG PLATEAU 3 DAMATURU DTR YB 6 ARM Pension Managers Limited 0026 S/N LGA NAME LGA STATE 12 HAWUL HWL BO 14 OVIA-NORTH-EAST AKA ED 26 26 OWERRI-NORTH RRT IM 38 TOFA TEA KN 4 EKITI ARP KW 15 OBAFEMI OWODE WDE OG S/N LGA NAME LGA STATE 4 FIKA FKA YB 7 Trustfund Pensions Plc 0028 CODE CODE 13 JERE JRE BO 15 OVIA-SOUTH-WEST GBZ ED 27 27 OWERRI-WEST UMG IM 39 TSANYAWA TYW KN 5 IFELODUN SHA KW 16 ODEDAH DED OG CODE CODE 5 FUNE FUN YB 8 First Guarantee Pension Limited 0029 1 AGUATA AGU AN 14 KAGA KGG BO 16 OWAN-EAST -

Small Holder Fish Farmer's Information and Training Needs In
Global Journal of Science Frontier Research: D Agriculture and Veterinary Volume 14 Issue 3 Version 1.0 Year 2014 Type : Double Blind Peer Reviewed International Research Journal Publisher: Global Journals Inc. (USA) Online ISSN: 2249-4626 & Print ISSN: 0975-5896 Small Holder Fish Farmer’s Information and Training Needs in Ogun State of Nigeria By O. J. Olaoye, S. S. Ashley-Dejo & Adekoya, E. O Federal University of Agriculture, Nigeria Abstract- The study was carried out to examine the small holder fish farmer’s information and training needs in Ogun State of Nigeria. Simple random sampling was used to select forty (40) fish farmers from each of the selected four agricultural extension zones. These data were collected from field through the use of structured interview; data obtained was subjected to descriptive statistics and inferential statistics. The study revealed that majority of the respondents fell between the age bracket of 41 and 50 years, over 60.0% were males and were married, with an household size ranges between 4 - 6 persons on average of 5 persons per house hold. The result indicated that majority of fish farmers sold fishes above N300:00 in all the four extension zones. The study show that majority of the respondents in the four agricultural extension zones performed fish farming management practices (cleaning, weeding, water quality) maintenance more frequently. Also fish production constraints faced includes high cost of feed, farm microcredit procurement and inadequate capital. The result of the hypotheses (ANOVA) revealed that there is a significant difference between the socioeconomic characteristics othe fish farmers and thier information and training needs at p<0.05 except for sex which showed a significant difference.Based on the research, more extension workers should be employed to give the technical knowledge to fish farmers on how to use some equipment and dissemination of new innovations on how to improve their fish farming system and productivity. -

Independent National Electoral Commission (INEC)
FEDERAL REPUBLIC OF NIGERIA Independent National Electoral Commission (INEC) OGUN STATE DIRECTORY OF POLLING UNITS Revised January 2015 DISCLAIMER The contents of this Directory should not be referred to as a legal or administrative document for the purpose of administrative boundary or political claims. Any error of omission or inclusion found should be brought to the attention of the Independent National Electoral Commission. INEC Nigeria Directory of Polling Units Revised January 2015 Page i Table of Contents Pages Disclaimer.............................................................................. i Table of Contents ………………………………………………. ii Foreword................................................................................ iv Acknowledgement.................................................................. v Summary of Polling Units....................................................... 1 LOCAL GOVERNMENT AREAS Abeokuta North............................................................. 2-11 Abeokuta South............................................................ 12-24 Ado Odo/Ota................................................................. 25-37 Egbado North................................................................ 38-46 Egbado South............................................................... 47-54 Ewekoro........................................................................ 55-61 Ifo.................................................................................. 62-70 Ijebu East..................................................................... -

Ogun State Statistical Year Book 2017 Edition
OGUN STATE STATISTICAL YEAR BOOK 2017 EDITION Central Department of Statistics, Ministry of Finance, Abeokuta, Ogun State, Nigeria. FOREWORD This is the fifth edition of the Ogun State Statistical Year Book. It consists of eleven sections; Weather and Climate, Population, Human Resources, Agriculture, Motor Vehicle, Medical and Health, Justice and Crime, Public Finance, Education, Price and Political Profile. Explanatory notes are given in the foot notes or in the introduction to each chapter, where necessary. Suggestions for the improvement of this publication are readily welcome from its users. It is believed that such suggestions, where acceptable shall be reflected in future editions. The Ogun State Statistical Year Book is subject to expansion as new information becomes available. Since the need for statistical data continues to arise from time to time, users are urged to make their data requirements known, with a view to having such needs met in subsequent editions. Director of Statistics Central Department of Statistics Ministry of Finance Abeokuta Ogun State October, 2018. i ACKNOWLEDGEMENT We express our gratitude to Federal and State Ministries, Departments and Agencies and all the Local Government Councils that supplied data for the production of this publication. Also, our appreciation goes to the United Nations Population Fund (UNFPA) for making the production of this publication possible. Director of Statistics Central Department of Statistics Ministry of Finance Abeokuta Ogun State October, 2017. ii TABLE OF CONTENT Title -

Comparative Analysis of Socio-Economic and Cultural Characteristics of Residents on Housing Patterns in International Border Towns of Ogun State, Nigeria
Proceedings of SOCIOINT 2019- 6th International Conference on Education, Social Sciences and Humanities 24-26 June 2019- Istanbul, Turkey COMPARATIVE ANALYSIS OF SOCIO-ECONOMIC AND CULTURAL CHARACTERISTICS OF RESIDENTS ON HOUSING PATTERNS IN INTERNATIONAL BORDER TOWNS OF OGUN STATE, NIGERIA OJO Oladimeji Olusola1*, OPOKO Akunnaya Pearl2, OLOTUAH Abiodun Olukayode3, OLUWATAYO Adedapo Adewunmi4 1PhD Student, Department of Architecture, Covenant University, Ota Ogun State, Nigeria, [email protected] 2Department of Architecture, Covenant University, Ota Ogun State, Nigeria, [email protected] 3Department of Architecture, Federal University of Technology, Akure Ondo State, Nigeria, [email protected] 4 Department of Architecture, Covenant University, Ota Ogun State, Nigeria, [email protected] *Corresponding Author Abstract Appropriate housing policy that enhances quality and standard of living in international border towns requires understanding the socio-economic and cultural factors of residents in the selected towns. However, research to this effect in international border between Nigeria and Benin Republic is either scarce or not available; hence the crux of the current study. Three towns at Nigeria-Benin Republic international border, namely Idi- Iroko, located within Ipokia Local Government Areas (LGA), Ohunbe town is in Yewa North LGA, while Ilara, falls within the jurisdiction of Imeko Afon LGA. These three towns are within the three LGAs in Ogun State that share border with the Benin Republic. It should be noted that an adequate study of these three border towns was considered as part of the research in order to draw a logical conclusion on variables germane to the study. The three towns were purposively selected given their high population sizes, location of government agencies and concentration of economic activities. -

1 11. No. 23 2012 12. Ogun State of Nigeria 13
1. HOUSE OF ASSEMBLY 2. OGUN STATE OF NIGERIA 3. THE SEVENTH LEGISLATURE (2011-2015) 4. ENROLLED HOUSE BILL NO. 23/OG/2012 5. SPONSOR: HON. BANKOLE, OLUSOLA AKANBI 6. CO-SPONSORS: HON. OBAFEMI, JOHN 7. HON. ADELEYE-OLADAPO,TUNRAYO (MRS) 8. HON. AKINTAN, JOB OLUFEMI 9. HON. FASANYA, VICTOR OLUDOTUN 10. HON. SOLAJA, O. BOWALE 11. NO. 23 2012 12. OGUN STATE OF NIGERIA 13. SENATOR IBIKUNLE AMOSUN, FCA 14. Governor of Ogun State 15. A BILL FOR A LAW TO AUTHORIZE THE ISSUE AND 16. APPROPRIATION OF THE SUM OF TWO HUNDRED AND 17. ELEVEN BILLION, SEVEN HUNDRED AND EIGHTY-NINE 18. MILLION, EIGHT HUNDRED AND SIX THOUSAND, 19. EIGHTY-EIGHT NAIRA, FIFTY-ONE KOBO ONLY, FROM 20. THE CONSOLIDATED REVENUE FUND FOR THE 21. SERVICES OF OGUN STATE GOVERNMENT OF NIGERIA, 22. FOR THE FINANCIAL YEAR ENDING THIRTY-FIRST DAY 23. OF DECEMBER, TWO THOUSAND AND THIRTEEN 24. (__________________________________) Date of Commencement 25. BE IT ENACTED by the House of Assembly of Ogun State 26. 1. This Law may be cited as the Year 2013 Appropriation Law. Short Title 27. 2. "The Accountant-General of the State may, when authorized The Issue and Appropriation of 28. to do so by warrants signed by the State Commissioner for N211,789,806,088.51 for 29. Finance, pay out of the Consolidated Revenue Fund of the Recurrent Expenditure and Capital Expenditure 30. State during the year ending 31st December, 2013, the sum from Consolidated Revenue Fund Charges 31. specified by warrants not exceeding in the aggregate, Two for the year ending 31st 32.