Synaptic Pathways Related to Shank3 and Its Interaction Partner Prosapip1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Regulation of Cdc42 and Its Effectors in Epithelial Morphogenesis Franck Pichaud1,2,*, Rhian F
© 2019. Published by The Company of Biologists Ltd | Journal of Cell Science (2019) 132, jcs217869. doi:10.1242/jcs.217869 REVIEW SUBJECT COLLECTION: ADHESION Regulation of Cdc42 and its effectors in epithelial morphogenesis Franck Pichaud1,2,*, Rhian F. Walther1 and Francisca Nunes de Almeida1 ABSTRACT An overview of Cdc42 Cdc42 – a member of the small Rho GTPase family – regulates cell Cdc42 was discovered in yeast and belongs to a large family of small – polarity across organisms from yeast to humans. It is an essential (20 30 kDa) GTP-binding proteins (Adams et al., 1990; Johnson regulator of polarized morphogenesis in epithelial cells, through and Pringle, 1990). It is part of the Ras-homologous Rho subfamily coordination of apical membrane morphogenesis, lumen formation and of GTPases, of which there are 20 members in humans, including junction maturation. In parallel, work in yeast and Caenorhabditis elegans the RhoA and Rac GTPases, (Hall, 2012). Rho, Rac and Cdc42 has provided important clues as to how this molecular switch can homologues are found in all eukaryotes, except for plants, which do generate and regulate polarity through localized activation or inhibition, not have a clear homologue for Cdc42. Together, the function of and cytoskeleton regulation. Recent studies have revealed how Rho GTPases influences most, if not all, cellular processes. important and complex these regulations can be during epithelial In the early 1990s, seminal work from Alan Hall and his morphogenesis. This complexity is mirrored by the fact that Cdc42 can collaborators identified Rho, Rac and Cdc42 as main regulators of exert its function through many effector proteins. -

1 Running Head
medRxiv preprint doi: https://doi.org/10.1101/2020.09.16.20194639; this version posted August 3, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . RUNNING HEAD: Alcohol use disorder dual methylation study Dual methylation and hydroxymethylation study of alcohol use disorder Shaunna L. Clark1, Robin F. Chan2, Min Zhao2, Lin Y. Xie2, William E. Copeland3, Brenda W.J.H. Penninx3, Karolina A. Aberg2, Edwin J.C.G. van den Oord2 1Department of Psychiatry, Texas A&M University 2Center for Biomarker Research and Precision Medicine, Virginia Commonwealth University 3 Department of Psychiatry, University of Vermont 4 Department of Psychiatry, Amsterdam UMC, Vrije Universiteit, Amsterdam, The Netherlands Corresponding Author: Shaunna L. Clark, Email: [email protected]; Address: 8441 Riverside Parkway, Bryan, TX, 77807; Phone: (979) 436-0179 NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. 1 medRxiv preprint doi: https://doi.org/10.1101/2020.09.16.20194639; this version posted August 3, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . ABSTRACT Using an integrative, multi-tissue design we sought to characterize methylation and hydroxymethylation changes in blood and brain associated with alcohol use disorder (AUD). -

Deciphering the Molecular Profile of Plaques, Memory Decline And
ORIGINAL RESEARCH ARTICLE published: 16 April 2014 AGING NEUROSCIENCE doi: 10.3389/fnagi.2014.00075 Deciphering the molecular profile of plaques, memory decline and neuron loss in two mouse models for Alzheimer’s disease by deep sequencing Yvonne Bouter 1†,Tim Kacprowski 2,3†, Robert Weissmann4, Katharina Dietrich1, Henning Borgers 1, Andreas Brauß1, Christian Sperling 4, Oliver Wirths 1, Mario Albrecht 2,5, Lars R. Jensen4, Andreas W. Kuss 4* andThomas A. Bayer 1* 1 Division of Molecular Psychiatry, Georg-August-University Goettingen, University Medicine Goettingen, Goettingen, Germany 2 Department of Bioinformatics, Institute of Biometrics and Medical Informatics, University Medicine Greifswald, Greifswald, Germany 3 Department of Functional Genomics, Interfaculty Institute for Genetics and Functional Genomics, University Medicine Greifswald, Greifswald, Germany 4 Human Molecular Genetics, Department for Human Genetics of the Institute for Genetics and Functional Genomics, Institute for Human Genetics, University Medicine Greifswald, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany 5 Institute for Knowledge Discovery, Graz University of Technology, Graz, Austria Edited by: One of the central research questions on the etiology of Alzheimer’s disease (AD) is the Isidro Ferrer, University of Barcelona, elucidation of the molecular signatures triggered by the amyloid cascade of pathological Spain events. Next-generation sequencing allows the identification of genes involved in disease Reviewed by: Isidro Ferrer, University of Barcelona, processes in an unbiased manner. We have combined this technique with the analysis of Spain two AD mouse models: (1) The 5XFAD model develops early plaque formation, intraneu- Dietmar R. Thal, University of Ulm, ronal Ab aggregation, neuron loss, and behavioral deficits. (2)TheTg4–42 model expresses Germany N-truncated Ab4–42 and develops neuron loss and behavioral deficits albeit without plaque *Correspondence: formation. -
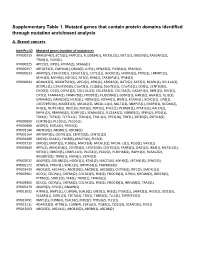
Supplementary Table 1. Mutated Genes That Contain Protein Domains Identified Through Mutation Enrichment Analysis
Supplementary Table 1. Mutated genes that contain protein domains identified through mutation enrichment analysis A. Breast cancers InterPro ID Mutated genes (number of mutations) IPR000219 ARHGEF4(2), ECT2(1), FARP1(1), FLJ20184(1), MCF2L2(1), NET1(1), OBSCN(5), RASGRF2(2), TRAD(1), VAV3(1) IPR000225 APC2(2), JUP(1), KPNA5(2), SPAG6(1) IPR000357 ARFGEF2(2), CMYA4(1), DRIM(2), JUP(1), KPNA5(2), PIK3R4(1), SPAG6(1) IPR000533 AKAP9(2), C10orf39(1), C20orf23(1), CUTL1(1), HOOK1(1), HOOK3(1), KTN1(2), LRRFIP1(3), MYH1(3), MYH9(2), NEF3(1), NF2(1), RSN(1), TAX1BP1(1), TPM4(1) IPR000694 ADAM12(3), ADAMTS19(1), APC2(2), APXL(1), ARID1B(1), BAT2(2), BAT3(1), BCAR1(1), BCL11A(2), BCORL1(1), C14orf155(3), C1orf2(1), C1QB(1), C6orf31(1), C7orf11(1), CD2(1), CENTD3(3), CHD5(3), CIC(3), CMYA1(2), COL11A1(3), COL19A1(2), COL7A1(3), DAZAP1(1), DBN1(3), DVL3(1), EIF5(1), FAM44A(1), FAM47B(1), FHOD1(1), FLJ20584(1), G3BP2(2), GAB1(2), GGA3(1), GLI1(3), GPNMB(2), GRIN2D(3), HCN3(1), HOXA3(2), HOXA4(1), IRS4(1), KCNA5(1), KCNC2(1), LIP8(1), LOC374955(1), MAGEE1(2), MICAL1(2), MICAL‐L1(1), MLLT2(1), MMP15(1), N4BP2(1), NCOA6(2), NHS(1), NUP214(3), ODZ1(3), PER1(2), PER2(1), PHC1(1), PLXNB1(1), PPM1E(2), RAI17(2), RAPH1(2), RBAF600(2), SCARF2(1), SEMA4G(1), SLC16A2(1), SORBS1(1), SPEN(2), SPG4(1), TBX1(1), TCF1(2), TCF7L1(1), TESK1(1), THG‐1(1), TP53(18), TRIF(1), ZBTB3(2), ZNF318(2) IPR000909 CENTB1(2), PLCB1(1), PLCG1(1) IPR000998 AEGP(3), EGFL6(2), PRSS7(1) IPR001140 ABCB10(2), ABCB6(1), ABCB8(2) IPR001164 ARFGAP3(1), CENTB1(2), CENTD3(3), CENTG1(2) IPR001589 -

The Human Gene Connectome As a Map of Short Cuts for Morbid Allele Discovery
The human gene connectome as a map of short cuts for morbid allele discovery Yuval Itana,1, Shen-Ying Zhanga,b, Guillaume Vogta,b, Avinash Abhyankara, Melina Hermana, Patrick Nitschkec, Dror Friedd, Lluis Quintana-Murcie, Laurent Abela,b, and Jean-Laurent Casanovaa,b,f aSt. Giles Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, NY 10065; bLaboratory of Human Genetics of Infectious Diseases, Necker Branch, Paris Descartes University, Institut National de la Santé et de la Recherche Médicale U980, Necker Medical School, 75015 Paris, France; cPlateforme Bioinformatique, Université Paris Descartes, 75116 Paris, France; dDepartment of Computer Science, Ben-Gurion University of the Negev, Beer-Sheva 84105, Israel; eUnit of Human Evolutionary Genetics, Centre National de la Recherche Scientifique, Unité de Recherche Associée 3012, Institut Pasteur, F-75015 Paris, France; and fPediatric Immunology-Hematology Unit, Necker Hospital for Sick Children, 75015 Paris, France Edited* by Bruce Beutler, University of Texas Southwestern Medical Center, Dallas, TX, and approved February 15, 2013 (received for review October 19, 2012) High-throughput genomic data reveal thousands of gene variants to detect a single mutated gene, with the other polymorphic genes per patient, and it is often difficult to determine which of these being of less interest. This goes some way to explaining why, variants underlies disease in a given individual. However, at the despite the abundance of NGS data, the discovery of disease- population level, there may be some degree of phenotypic homo- causing alleles from such data remains somewhat limited. geneity, with alterations of specific physiological pathways under- We developed the human gene connectome (HGC) to over- come this problem. -

Rare Gene Deletions in Genetic Generalized and Rolandic Epilepsies
RESEARCH ARTICLE Rare gene deletions in genetic generalized and Rolandic epilepsies Kamel Jabbari1,2☯, Dheeraj R. Bobbili3☯, Dennis Lal1,4,5,6, Eva M. Reinthaler7, Julian Schubert8, Stefan Wolking8, Vishal Sinha9, Susanne Motameny1, Holger Thiele1, Amit Kawalia1, Janine AltmuÈ ller1,10, Mohammad Reza Toliat1, Robert Kraaij11, Jeroen van Rooij11, Andre G. Uitterlinden11, M. Arfan Ikram12, EuroEPINOMICS CoGIE Consortium¶, Federico Zara13, Anna-Elina Lehesjoki14,15, Roland Krause3, Fritz Zimprich7, Thomas Sander1, Bernd A. Neubauer16, Patrick May3, Holger Lerche8, Peter NuÈrnberg1,17,18* a1111111111 1 Cologne Center for Genomics, University of Cologne, Cologne, Germany, 2 Cologne Biocenter, Institute a1111111111 for Genetics, University of Cologne, Cologne, Germany, 3 Luxembourg Centre for Systems Biomedicine, a1111111111 University of Luxembourg, Esch-sur-Alzette, Luxembourg, 4 Psychiatric and Neurodevelopmental Genetics a1111111111 Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, United States of a1111111111 America, 5 Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, United States of America, 6 Stanley Center for Psychiatric Research, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, United States of America, 7 Department of Neurology, Medical University of Vienna, Vienna, Austria, 8 Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research, University of TuÈbingen, TuÈbingen, Germany, 9 Institute for Molecular Medicine FIMM, University of Helsinki, Helsinki, Finland, 10 Institute of Human Genetics, University of Cologne, OPEN ACCESS Cologne, Germany, 11 Department of Internal Medicine, Erasmus Medical Center, Rotterdam, the Netherlands, 12 Departments of Epidemiology, Neurology, and Radiology, Erasmus Medical Center, Citation: Jabbari K, Bobbili DR, Lal D, Reinthaler Rotterdam, The Netherlands, 13 Laboratory of Neurogenetics and Neuroscience, Institute G. -

Mouse Baiap2 Knockout Project (CRISPR/Cas9)
https://www.alphaknockout.com Mouse Baiap2 Knockout Project (CRISPR/Cas9) Objective: To create a Baiap2 knockout Mouse model (C57BL/6J) by CRISPR/Cas-mediated genome engineering. Strategy summary: The Baiap2 gene (NCBI Reference Sequence: NM_130862 ; Ensembl: ENSMUSG00000025372 ) is located on Mouse chromosome 11. 14 exons are identified, with the ATG start codon in exon 1 and the TGA stop codon in exon 14 (Transcript: ENSMUST00000075180). Exon 2~3 will be selected as target site. Cas9 and gRNA will be co-injected into fertilized eggs for KO Mouse production. The pups will be genotyped by PCR followed by sequencing analysis. Note: Homozygotes for a knock-out allele show mid to late gestation lethality, developmental delay, oligodactyly, subcutaneous edema, and severely impaired cardiac and placental development. Adult homozygotes fail to regulate synaptic plasticity and exhibit hippocampus-associated learning deficits. Exon 2 starts from about 3.51% of the coding region. Exon 2~3 covers 10.41% of the coding region. The size of effective KO region: ~3142 bp. The KO region does not have any other known gene. Page 1 of 9 https://www.alphaknockout.com Overview of the Targeting Strategy Wildtype allele 5' gRNA region gRNA region 3' 1 2 3 14 Legends Exon of mouse Baiap2 Knockout region Page 2 of 9 https://www.alphaknockout.com Overview of the Dot Plot (up) Window size: 15 bp Forward Reverse Complement Sequence 12 Note: The 2000 bp section upstream of Exon 2 is aligned with itself to determine if there are tandem repeats. No significant tandem repeat is found in the dot plot matrix. -

A 29 Mainland Chinese Cohort of Patients with Phelan–Mcdermid
Xu et al. Orphanet J Rare Dis (2020) 15:335 https://doi.org/10.1186/s13023-020-01592-5 RESEARCH Open Access A 29 Mainland Chinese cohort of patients with Phelan–McDermid syndrome: genotype– phenotype correlations and the role of SHANK3 haploinsufciency in the important phenotypes Na Xu1†, Hui Lv2†, Tingting Yang1†, Xiujuan Du2, Yu Sun1, Bing Xiao1, Yanjie Fan1, Xiaomei Luo1, Yongkun Zhan1, Lili Wang1, Fei Li2* and Yongguo Yu1,3* Abstract Background: Phelan–McDermid syndrome (PMS) or 22q13 deletion syndrome is a rare developmental disorder characterized by hypotonia, developmental delay (DD), intellectual disability (ID), autism spectrum disorder (ASD) and dysmorphic features. Most cases are caused by 22q13 deletions encompassing many genes including SHANK3. Phenotype comparisons between patients with SHANK3 mutations (or deletions only disrupt SHANK3) and 22q13 deletions encompassing more than SHANK3 gene are lacking. Methods: A total of 29 Mainland China patients were clinically and genetically evaluated. Data were obtained from medical record review and a standardized medical history questionnaire, and dysmorphology evaluation was conducted via photographic evaluation. We analyzed 22q13 deletions and SHANK3 small mutations and performed genotype–phenotype analysis to determine whether neurological features and other important clinical features are responsible for haploinsufciency of SHANK3. Results: Nineteen patients with 22q13.3 deletions ranging in size from 34 kb to 8.7 Mb, one patient with terminal deletions and duplications, and nine patients with SHANK3 mutations were included. All mutations would cause loss-of function efect and six novel heterozygous variants, c.3838_3839insGG, c.3088delC, c.3526G > T, c.3372dupC, c.3120delC and c.3942delC, were frstly reported. -

Whole Genome Sequencing Puts Forward Hypotheses on Metastasis Evolution and Therapy in Colorectal Cancer
ARTICLE DOI: 10.1038/s41467-018-07041-z OPEN Whole genome sequencing puts forward hypotheses on metastasis evolution and therapy in colorectal cancer Naveed Ishaque1,2,3, Mohammed L. Abba4,5, Christine Hauser4,5, Nitin Patil4,5, Nagarajan Paramasivam3,6, Daniel Huebschmann 3, Jörg Hendrik Leupold4,5, Gnana Prakash Balasubramanian2, Kortine Kleinheinz 3, Umut H. Toprak3, Barbara Hutter 2, Axel Benner7, Anna Shavinskaya4, Chan Zhou4,5, Zuguang Gu1,3, Jules Kerssemakers 3, Alexander Marx8, Marcin Moniuszko9, Miroslaw Kozlowski9, Joanna Reszec9, Jacek Niklinski9, Jürgen Eils3, Matthias Schlesner 3,10, Roland Eils 1,3,11, Benedikt Brors 2,12 & 1234567890():,; Heike Allgayer 4,5 Incomplete understanding of the metastatic process hinders personalized therapy. Here we report the most comprehensive whole-genome study of colorectal metastases vs. matched primary tumors. 65% of somatic mutations originate from a common progenitor, with 15% being tumor- and 19% metastasis-specific, implicating a higher mutation rate in metastases. Tumor- and metastasis-specific mutations harbor elevated levels of BRCAness. We confirm multistage progression with new components ARHGEF7/ARHGEF33. Recurrently mutated non-coding elements include ncRNAs RP11-594N15.3, AC010091, SNHG14,3’ UTRs of FOXP2, DACH2, TRPM3, XKR4, ANO5, CBL, CBLB, the latter four potentially dual protagonists in metastasis and efferocytosis-/PD-L1 mediated immunosuppression. Actionable metastasis- specific lesions include FAT1, FGF1, BRCA2, KDR, and AKT2-, AKT3-, and PDGFRA-3’ UTRs. Metastasis specific mutations are enriched in PI3K-Akt signaling, cell adhesion, ECM and hepatic stellate activation genes, suggesting genetic programs for site-specific colonization. Our results put forward hypotheses on tumor and metastasis evolution, and evidence for metastasis-specific events relevant for personalized therapy. -

Genetic Findings As the Potential Basis of Personalized Pharmacotherapy in Phelan-Mcdermid Syndrome
G C A T T A C G G C A T genes Review Genetic Findings as the Potential Basis of Personalized Pharmacotherapy in Phelan-McDermid Syndrome Brianna Dyar 1, Erika Meaddough 1, Sara M. Sarasua 1, Curtis Rogers 2, Katy Phelan 3 and Luigi Boccuto 1,* 1 Healthcare Genetics Program, School of Nursing, Clemson University, Clemson, SC 29634, USA; [email protected] (B.D.); [email protected] (E.M.); [email protected] (S.M.S.) 2 Greenwood Genetic Center, Greenwood, SC 29649, USA; [email protected] 3 Florida Cancer Specialists & Research Institute, Fort Myers, FL 33905, USA; kphelan@flcancer.com * Correspondence: [email protected] Abstract: Phelan-McDermid syndrome (PMS) is a genetic disorder often characterized by autism or autistic-like behavior. Most cases are associated with haploinsufficiency of the SHANK3 gene resulting from deletion of the gene at 22q13.3 or from a pathogenic variant in the gene. Treatment of PMS often targets SHANK3, yet deletion size varies from <50 kb to >9 Mb, potentially encompassing dozens of genes and disrupting regulatory elements altering gene expression, inferring the potential for multiple therapeutic targets. Repurposed drugs have been used in clinical trials investigating therapies for PMS: insulin-like growth factor 1 (IGF-1) for its effect on social and aberrant behaviors, intranasal insulin for improvements in cognitive and social ability, and lithium for reversing regression and stabilizing behavior. The pharmacogenomics of PMS is complicated by the CYP2D6 enzyme which metabolizes antidepressants and antipsychotics often used for treatment. The gene coding for Citation: Dyar, B.; Meaddough, E.; CYP2D6 maps to 22q13.2 and is lost in individuals with deletions larger than 8 Mb. -

Perkinelmer Genomics to Request the Saliva Swab Collection Kit for Patients That Cannot Provide a Blood Sample As Whole Blood Is the Preferred Sample
Autism and Intellectual Disability TRIO Panel Test Code TR002 Test Summary This test analyzes 2429 genes that have been associated with Autism and Intellectual Disability and/or disorders associated with Autism and Intellectual Disability with the analysis being performed as a TRIO Turn-Around-Time (TAT)* 3 - 5 weeks Acceptable Sample Types Whole Blood (EDTA) (Preferred sample type) DNA, Isolated Dried Blood Spots Saliva Acceptable Billing Types Self (patient) Payment Institutional Billing Commercial Insurance Indications for Testing Comprehensive test for patients with intellectual disability or global developmental delays (Moeschler et al 2014 PMID: 25157020). Comprehensive test for individuals with multiple congenital anomalies (Miller et al. 2010 PMID 20466091). Patients with autism/autism spectrum disorders (ASDs). Suspected autosomal recessive condition due to close familial relations Previously negative karyotyping and/or chromosomal microarray results. Test Description This panel analyzes 2429 genes that have been associated with Autism and ID and/or disorders associated with Autism and ID. Both sequencing and deletion/duplication (CNV) analysis will be performed on the coding regions of all genes included (unless otherwise marked). All analysis is performed utilizing Next Generation Sequencing (NGS) technology. CNV analysis is designed to detect the majority of deletions and duplications of three exons or greater in size. Smaller CNV events may also be detected and reported, but additional follow-up testing is recommended if a smaller CNV is suspected. All variants are classified according to ACMG guidelines. Condition Description Autism Spectrum Disorder (ASD) refers to a group of developmental disabilities that are typically associated with challenges of varying severity in the areas of social interaction, communication, and repetitive/restricted behaviors. -

Indications of Linkage and Association of Gilles De La Tourette Syndrome in Two Independent Family Samples: 17Q25 Is a Putative Susceptibility Region P
Am. J. Hum. Genet. 75:545–560, 2004 Indications of Linkage and Association of Gilles de la Tourette Syndrome in Two Independent Family Samples: 17q25 Is a Putative Susceptibility Region P. Paschou,1 Y. Feng,2 A. J. Pakstis,1 W. C. Speed,1 M. M. DeMille,1 J. R. Kidd,1 B. Jaghori,2 R. Kurlan,5 D. L. Pauls,6 P. Sandor,2,3 C. L. Barr,2,4 and K. K. Kidd1 1Department of Genetics, Yale University School of Medicine, New Haven; 2Toronto Western Research Institute, University Health Network, 3Department of Psychiatry, University of Toronto, and 4Department of Psychiatry, Brain and Behaviour Programme, The Hospital for Sick Children, Toronto; 5Cognitive and Behavioral Neurology Unit, Department of Neurology, University of Rochester School of Medicine, Rochester, NY; and 6Psychiatric and Neurodevelopmental Genetics Unit, Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA Gilles de la Tourette syndrome (GTS) is characterized by multiple motor and phonic tics and high comorbidity rates with other neurobehavioral disorders. It is hypothesized that frontal-subcortical pathways and a complex genetic background are involved in the etiopathogenesis of the disorder. The genetic basis of GTS remains elusive. However, several genomic regions have been implicated. Among them, 17q25 appears to be of special interest, as suggested by various independent investigators. In the present study, we explored the possibility that 17q25 con- tributes to the genetic component of GTS. The initial scan of chromosome 17 performed on two large pedigrees provided a nonparametric LOD score of 2.41 near D17S928. Fine mapping with 17 additional microsatellite markers increased the peak to 2.61 (P p .002 ).