An Investigation of Growth Rotations of the Jaws
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A New Dimension of Success in Your Practice
3D Imaging Family A new dimension of success in your practice dentsplysirona.com CEREC® Diagnosis Treatment Plan Guided Endodontics Airway Functional Orthodontics Integration Implantology Analysis Occlusal & TMD 2/3 Good reasons for 3D With 3D imaging, you have the ideal basis for a new dimension of success in your practice. Best image quality at a low dose and shorter visits—that is what Dentsply Sirona 3D X-ray units provide for your practice. These BETTER benefits provide greater certainty to help make difcult diagnoses Communicate with easier, while providing the opportunity to explore new options for stunning images implantology, endodontics, orthodontics, and more. to your patients Thanks to the 3D Family, Galileos® Comfort Plus, Orthophos® SL 3D and Orthophos XG 3D patients have a better understanding of the diagnosis and accept treatment more readily. It all adds up to efcient clinical workflow that leads to greater practice success. Enjoy every day. With Dentsply Sirona. SAFER Predictable diagnosis and treatment options FASTER Efcient clinical workflow 4/5 More insight More possibilities Your patients are candidates for 3D more often than you think. How severe is the bone atrophy or the periapical lesion? Is the tooth impacted? In all dental disciplines, there are numerous questions that can be answered far more easily using 3D imaging with CBCT. 3D CBCT from Dentsply Sirona ofers clinicians and specialists numerous When does 3D provide more certainty? options for diagnosis, treatment plans, patient consultation—all with a seamless, efcient workflow. This is one way you can expand your range Areas Cases of services and treat more patients at your practice. -

Viewing in 3D
Viewing in 3D Viewing in 3D Foley & Van Dam, Chapter 6 • Transformation Pipeline • Viewing Plane • Viewing Coordinate System • Projections • Orthographic • Perspective OpenGL Transformation Pipeline Viewing Coordinate System Homogeneous coordinates in World System zw world yw ModelViewModelView Matrix Matrix xw Tractor Viewing System Viewer Coordinates System ProjectionProjection Matrix Matrix Clip y Coordinates v Front- xv ClippingClipping Wheel System P0 zv ViewportViewport Transformation Transformation ne pla ing Window Coordinates View Specifying the Viewing Coordinates Specifying the Viewing Coordinates • Viewing Coordinates system, [xv, yv, zv], describes 3D objects with respect to a viewer zw y v P v xv •A viewing plane (projection plane) is set up N P0 zv perpendicular to zv and aligned with (xv,yv) yw xw ne pla ing • In order to specify a viewing plane we have View to specify: •P0=(x0,y0,z0) is the point where a camera is located •a vector N normal to the plane • P is a point to look-at •N=(P-P)/|P -P| is the view-plane normal vector •a viewing-up vector V 0 0 •V=zw is the view up vector, whose projection onto • a point on the viewing plane the view-plane is directed up Viewing Coordinate System Projections V u N z N ; x ; y z u x • Viewing 3D objects on a 2D display requires a v v V u N v v v mapping from 3D to 2D • The transformation M, from world-coordinate into viewing-coordinates is: • A projection is formed by the intersection of certain lines (projectors) with the view plane 1 2 3 ª x v x v x v 0 º ª 1 0 0 x 0 º « » « -

Class II Article
Journal of the World Federation of Orthodontists 4 (2015) 40e49 Contents lists available at ScienceDirect Journal of the World Federation of Orthodontists journal homepage: www.jwfo.org Case Report A new, no-compliance class II correction strategy using nickel-titanium coil-springs Luca Lombardo a,*, Antonella Carlucci b, Francesca Cervinara c, Giuseppe Siciliani d a Adjunct Professor, Postgraduate School of Orthodontics, University of Ferrara, Ferrara, Italy b Postgraduate Student, Postgraduate School of Orthodontics, University of Ferrara, Ferrara, Italy c Private Practice in Bari, Italy d Chairman of Postgraduate School of Orthodontics, Department of Orthodontics, University of Ferrara, Ferrara, Italy article info abstract Article history: Background: Correcting Class II malocclusion with Class II elastics or functional appliances requires great Received 15 October 2014 patient collaboration. Here we describe two Class II cases successfully treated with an alternative Received in revised form approach using a fixed device designed to obviate compliance. 27 November 2014 Methods: We fitted specific Class II springs to the bilateral hooks on the stainless steel maxillary and Accepted 3 December 2014 mandibular archwires of a full fixed appliance to correct the Class II malocclusion and to promote Available online 14 February 2015 mandibular growth. Results: The new device brought about full Class I canine and molar relationships in both treated cases Keywords: Class II and improved the maxillomandibular relationship without relying on patient collaboration. Compliance-free Conclusion: Class II springs appear to be a simple, fast, and effective alternative approach to Class II Spring correction, facilitating mandibular growth even in noncompliant patients. Ó 2015 World Federation of Orthodontists. -

Cephalometric and Malocclusion Analysis of Kadazan Dusun Ethnic Orthodontic Patients (Analisis Sefalometrik Dan Maloklusi Pesakit Ortodontik Etnik Kadazan Dusun)
Sains Malaysiana 42(1)(2013): 25–32 Cephalometric and Malocclusion Analysis of Kadazan Dusun Ethnic Orthodontic Patients (Analisis Sefalometrik dan Maloklusi Pesakit Ortodontik Etnik Kadazan Dusun) ROHAYA MEGAT ABDUL WAHAB* HARTINI IDRIS, HABIBAH YACOB & SHAHRUL HISHAM ZAINAL ARIFFIN ABSTRACT The aims of this study were to assess the skeletal pattern and the malocclusion of Kadazan Dusun ethnic patients who seek for orthodontic treatment. Cephalometric radiographs (248) and 345 study models were collected from four orthodontic clinics in Sabah. The cephalometric mean values (SNA, SNB, ANB, MMA, SNMxP, UIMxP, LIMnP and ALFH) were measured and the study models were analysed for overjet, overbite, incisor and molar relationships. Some morphological or occlusal features such as shovel shape, Talon cusp, peg shape teeth, midline diastema, canine displacement and supernumerary tooth were also noted. The frequency and correlation of cephalometric mean values and prevalence of malocclusion were analysed using SPSS 18. Class I Skeletal pattern was the most common (48%) followed by Class II (33%) and Class III (18%). There was a strong correlation between SNA and SNB values (>0.70). Class II/1 incisor relationship has the highest frequency (41%) followed by Class III (32%), Class I (21%) and Class II/2 (6%). Class II Molar relationship of both right and left showed highest frequency (38%) followed by Class I (33%) and Class III (30%). Increased of overjet (44%) and reduced overbite (41%) and shovel-shaped incisor were the most common occlusal and dental features. The Kadazan Dusun patients who seek for orthodontic treatment in Sabah were mostly presented with Class I Skeletal pattern with high prevalence in Class II/1 incisor relationship, Class II molar relationship, increased overjet and reduced overbite. -

The Frontal Cephalometric Analysis – the Forgotten Perspective
CONTINUING EDUCATION The frontal cephalometric analysis – the forgotten perspective Dr. Bradford Edgren delves into the benefits of the frontal analysis hen greeting a person for the first Wtime, we are supposed to make Educational aims and objectives This article aims to discuss the frontal cephalometric analysis and its direct eye contact and smile. But how often advantages in diagnosis. when you meet a person for the first time do you greet them towards the side of the Expected outcomes Correctly answering the questions on page xx, worth 2 hours of CE, will face? Nonetheless, this is generally the only demonstrate the reader can: perspective by which orthodontists routinely • Understand the value of the frontal analysis in orthodontic diagnosis. evaluate their patients radiographically • Recognize how the certain skeletal facial relationships can be detrimental to skeletal patterns that can affect orthodontic and cephalometrically. Rarely is a frontal treatment. radiograph and cephalometric analysis • Realize how frontal analysis is helpful for evaluation of skeletal facial made, even though our first impression of asymmetries. • Identify the importance of properly diagnosing transverse that new patient is from the front, when we discrepancies in all patients; especially the growing patient. greet him/her for the first time. • Realize the necessity to take appropriate, updated records on all A patient’s own smile assessment transfer patients. is made in the mirror, from the facial perspective. It is also the same perspective by which he/she will ultimately decide cephalometric analysis. outcomes. Furthermore, skeletal lingual if orthodontic treatment is a success Since all orthodontic patients are three- crossbite patterns are not just limited to or a failure. -

Map Projections
Map Projections Chapter 4 Map Projections What is map projection? Why are map projections drawn? What are the different types of projections? Which projection is most suitably used for which area? In this chapter, we will seek the answers of such essential questions. MAP PROJECTION Map projection is the method of transferring the graticule of latitude and longitude on a plane surface. It can also be defined as the transformation of spherical network of parallels and meridians on a plane surface. As you know that, the earth on which we live in is not flat. It is geoid in shape like a sphere. A globe is the best model of the earth. Due to this property of the globe, the shape and sizes of the continents and oceans are accurately shown on it. It also shows the directions and distances very accurately. The globe is divided into various segments by the lines of latitude and longitude. The horizontal lines represent the parallels of latitude and the vertical lines represent the meridians of the longitude. The network of parallels and meridians is called graticule. This network facilitates drawing of maps. Drawing of the graticule on a flat surface is called projection. But a globe has many limitations. It is expensive. It can neither be carried everywhere easily nor can a minor detail be shown on it. Besides, on the globe the meridians are semi-circles and the parallels 35 are circles. When they are transferred on a plane surface, they become intersecting straight lines or curved lines. 2021-22 Practical Work in Geography NEED FOR MAP PROJECTION The need for a map projection mainly arises to have a detailed study of a 36 region, which is not possible to do from a globe. -

189 09 Aju 03 Bryon 8/1/10 07:25 Página 31
189_09 aju 03 Bryon 8/1/10 07:25 Página 31 Measuring the qualities of Choisy’s oblique and axonometric projections Hilary Bryon Auguste Choisy is renowned for his «axonometric» representations, particularly those illustrating his Histoire de l’architecture (1899). Yet, «axonometric» is a misnomer if uniformly applied to describe Choisy’s pictorial parallel projections. The nomenclature of parallel projection is often ambiguous and confusing. Yet, the actual history of parallel projection reveals a drawing system delineated by oblique and axonometric projections which relate to inherent spatial differences. By clarifying the intrinsic demarcations between these two forms of parallel pro- jection, one can discern that Choisy not only used the two spatial classes of pictor- ial parallel projection, the oblique and the orthographic axonometric, but in fact manipulated their inherent differences to communicate his theory of architecture. Parallel projection is a form of pictorial representation in which the projectors are parallel. Unlike perspective projection, in which the projectors meet at a fixed point in space, parallel projectors are said to meet at infinity. Oblique and axonometric projections are differentiated by the directions of their parallel pro- jectors. Oblique projection is delineated by projectors oblique to the plane of pro- jection, whereas the orthographic axonometric projection is defined by projectors perpendicular to the plane of projection. Axonometric projection is differentiated relative to its angles of rotation to the picture plane. When all three axes are ro- tated so that each is equally inclined to the plane of projection, the axonometric projection is isometric; all three axes are foreshortened and scaled equally. -

2016-Chapter-143-Oropharyngeal-Growth-And-Malformations-PPSM-6E-1.Pdf
To protect the rights of the author(s) and publisher we inform you that this PDF is an uncorrected proof for internal business use only by the author(s), editor(s), reviewer(s), Elsevier and typesetter Toppan Best-set. It is not allowed to publish this proof online or in print. This proof copy is the copyright property of the publisher and is confidential until formal publication. Chapter c00143 Oropharyngeal Growth and Skeletal Malformations 143 Stacey Dagmar Quo; Benjamin T. Pliska; Nelly Huynh Chapter Highlights p0010 • Sleep-disordered breathing (SDB) is marked by manifestations has not been determined. The varying degrees of collapsibility of the length and volume of the airway increase until pharyngeal airway. The hard tissue boundaries the age of 20 years, at which time there is a of the airway dictate the size and therefore the variable period of stability, followed by a slow responsiveness of the muscles that form this decrease in airway size after the fifth decade of part of the upper airway. Thus, the airway is life. The possibility of addressing the early forms shaped not only by the performance of the of this disease with the notions of intervention pharyngeal muscles to stimulation but also by and prevention can change the landscape the surrounding skeletal framework. of care. u0015 • The upper and lower jaws are key components • Correction of specific skeletal anatomic u0025 of the craniofacial skeleton and the deficiencies can improve or eliminate SDB determinants of the anterior wall of the upper symptoms in both children and adults. It is airway. The morphology of the jaws can be possible that the clinician may adapt or modify negatively altered by dysfunction of the upper the growth expression, although the extent of airway during growth and development. -

Visual Impairment and Deafblind Education Quarterly Volume 61
Visual Impairment and Deafblind Education Quarterly 2016 Convention Issue Volume 61 Number 2 2016 ; Lorem Ipsum Dolor Spring 2016 This is a publication of the Council for Exceptional Children’s Division on Visual Impairments and DeafBlindness (CEC- DVIDB). Advertisements included in this issue are not endorsements of products or services, and individual views of authors are not necessarily the official position of CEC and/or DVIDB. Cover Photo Cover photo is of the St. Louis arch. St. Louis, Missouri was the home of CEC’s 2016 International Convention. Photograph courtesy of Stephanie Barrows. 2 2 ; Lorem Ipsum Dolor Spring 2016 Volume 61, Number 21 Page 6 Message from the Editor 8 President’s Message 14 Virgina M. Sowell Award: Jessica Kolvites 16 Dissertation of the Year Award: Dr. Ellen Bowman 19 Teacher of the Year Award: Rachel Schles 23 Exemplary Advocate Award: Teresa Lacy 26 Distinguished Service Award: Dr. Alana Zambone 30 Including Students Who Are Blind or Have Low Vision in English Language Proficiency Assessments 35 Early Intervention and Visual Impairments: A Prepared Workforce 53 Introducing the iBraille Challenge! 3 3 ; Lorem Ipsum Dolor Spring 2016 Volume 61, Number 2 Page 60 Mathematics Instruction for Students with Visual Impairments: What is there and where can we go 70 Peer Assisted Learning Strategies to Improve Reading Fluency and Socialization Among Students Who Are Blind and Visually Impaired 77 University and School for the Deaf and Blind Parternship Experience 79 The Expanded Core Curriculum: What We Learned at the Florida School for the Deaf and Blind 85 Experience of a Lifetime for an Undergraduate Student 92 Missouri School for the Blind 107 DVIDB Professional Standards Committee Report 4 4 ; Lorem Ipsum Dolor Spring 2016 Volume 61, Number 2 Page 109 Ad Hoc Committee on DVIDB Position Paper 116 The Expanded Core Curriculum 137 CEC Membership Application 5 5 ; Lorem Ipsum Dolor Spring 2016 Kathleen Farrand, Ph.D. -

Craniofacial Growth Nasal Septum Deviation
International Journal of Pediatric Otorhinolaryngology 74 (2010) 1180–1183 Contents lists available at ScienceDirect International Journal of Pediatric Otorhinolaryngology journal homepage: www.elsevier.com/locate/ijporl Craniofacial growth in children with nasal septum deviation: A cephalometric comparative study Luca D’Ascanio a,b,*, Carla Lancione a, Giorgio Pompa b, Elena Rebuffini c, Nicola Mansi d, Marco Manzini a a Department of Otolaryngology – Head & Neck Surgery, Citta` di Castello Civil Hospital, Citta` di Castello (Perugia), Italy b Department of Dentistry and Maxillo-Facial Surgery, ‘‘La Sapienza’’ University of Rome, Rome, Italy c Department of Maxillo-Facial Surgery, University of Ferrara, Ferrara, Italy d Department of Otolaryngology, ‘‘Santobono–Pausilipon’’ Children’s Hospital, Naples, Italy ARTICLE INFO ABSTRACT Article history: Objective: Nasal-breathing impairment has been described as a possible determinant of maxillofacial Received 2 April 2010 development in children with adenoids/tonsils hypertrophy. However little is known about the possible Received in revised form 13 July 2010 influence of nasal septum deviation on craniofacial growth in childhood. We conducted a multicenter Accepted 15 July 2010 cephalometric study to compare skeletal and dental features in children with chronic nasal-breathing Available online 9 August 2010 obstruction secondary to nasal septum deviation and nose-breathing controls. Methods: Ninety-eight children (59M, 39F; mean age 8.8 years; age range 7–12 years) with obligate Keywords: mouth-breathing secondary to nasal septum deviation (group 1) and 98 age- and sex-matched nasal- Craniofacial growth breathing controls (group 2) were evaluated. Nasal-breathing function was assessed in all patients with Nasal septum Childhood clinical history, ENT instrumental examination and anterior active rhinomanometry. -

Viewing and Projection Viewing and Projection
Viewing and Projection The topics • Interior parameters • Projection type • Field of view • Clipping • Frustum… • Exterior parameters • Camera position • Camera orientation Transformation Pipeline Local coordinate Local‐>World World coordinate ModelView World‐>Eye Matrix Eye coordinate Projection Matrix Clip coordina te others Screen coordinate Projection • The projection transforms a point from a high‐ dimensional space to a low‐dimensional space. • In 3D, the projection means mapping a 3D point onto a 2D projection plane (or called image plane). • There are two basic projection types: • Parallel: orthographic, oblique • Perspective Orthographic Projection Image Plane Direction of Projection z-axis z=k x 1000 x y 0100 y k 000k z 1 0001 1 Orthographic Projection Oblique Projection Image Plane Direction of Projection Properties of Parallel Projection • Definition: projection directions are parallel. • Doesn’t look real. • Can preserve parallel lines Projection PlllParallel in 3D PlllParallel in 2D Properties of Parallel Projection • Definition: projection directions are parallel. • Doesn’t look real. • Can preserve parallel lines • Can preserve ratios t ' t Projection s s :t s' :t ' s' Properties of Parallel Projection • Definition: projection directions are parallel. • Doesn’t look real. • Can preserve parallel lines • Can preserve ratios • CANNOT preserve angles Projection Properties of Parallel Projection • Definition: projection directions are parallel. • Doesn’t look real. • Can preserve parallel -
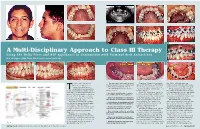
A Multi-Disciplinary Approach to Class III Therapy Using the Delta Force and ALF Appliances in Conjunction with Terminal Arch Extractions
Fig. 3 Fig. 9 Fig. 10 Fig. 11 Fig. 1 Fig. 2 Fig. 4 Fig. 14 Fig. 12 Fig. 13 A Multi-Disciplinary Approach to Class III Therapy Using The Delta Force and ALF Appliances in Conjunction with Terminal Arch Extractions By J. Wellington (Skip) Truitt, DDS & Livier Carreon-Truitt, DDS Fig. 15 Fig. 5 Fig. 6 Fig. 7 Fig. 16 Fig. 17 Fig. 18 he use of the low friction The panoramic radiograph showed Treatment began by placing an the .020 x .020 arch wire was Delta Force appliance in the third molars to be developing very upper ALF appliance on First Molar replaced every sixty days with a new conjunction with the bio- well in all four quadrants. (Fig. 9) Bands with standard pre-adjusted arch wire of the same type and gauge. compatible forces generated The following was the proposed .022 buccal tubes containing 10° of The treatment objective for this byT the ALF appliance creates a new treatment plan: torque. (Fig. 10) first phase of therapy was to develop approach to treating under-developed Composite ledges were placed on the maxillary arch over the mandibular • Develop the maxilla using a combina- arches. It is now possible to develop tion of ALF and Delta Force appliances. both the left and right maxillary 1st arch. Once this first phase of treat- the size of both the upper or lower bicuspids to secure the crescent wires ment was accomplished therapy could arch and level, align and torque the • Remove the mandibular second molars of the ALF appliance. (Fig. 11) All be initiated on the lower arch.