A Study to Design Minimum Data Set of COVID-19 Registry System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
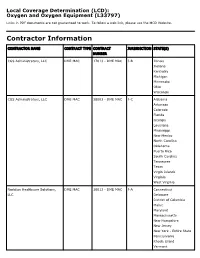
Oxygen and Oxygen Equipment Local Coverage Determination (LCD)
Local Coverage Determination (LCD): Oxygen and Oxygen Equipment (L33797) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information CONTRACTOR NAME CONTRACT TYPE CONTRACT JURISDICTION STATE(S) NUMBER CGS Administrators, LLC DME MAC 17013 - DME MAC J-B Illinois Indiana Kentucky Michigan Minnesota Ohio Wisconsin CGS Administrators, LLC DME MAC 18003 - DME MAC J-C Alabama Arkansas Colorado Florida Georgia Louisiana Mississippi New Mexico North Carolina Oklahoma Puerto Rico South Carolina Tennessee Texas Virgin Islands Virginia West Virginia Noridian Healthcare Solutions, DME MAC 16013 - DME MAC J-A Connecticut LLC Delaware District of Columbia Maine Maryland Massachusetts New Hampshire New Jersey New York - Entire State Pennsylvania Rhode Island Vermont CONTRACTOR NAME CONTRACT TYPE CONTRACT JURISDICTION STATE(S) NUMBER Noridian Healthcare Solutions, DME MAC 19003 - DME MAC J-D Alaska LLC American Samoa Arizona California - Entire State Guam Hawaii Idaho Iowa Kansas Missouri - Entire State Montana Nebraska Nevada North Dakota Northern Mariana Islands Oregon South Dakota Utah Washington Wyoming LCD Information Document Information LCD ID Original Effective Date L33797 For services performed on or after 10/01/2015 LCD Title Revision Effective Date Oxygen and Oxygen Equipment For services performed on or after 08/02/2020 Proposed LCD in Comment Period Revision Ending Date N/A N/A Source Proposed LCD Retirement Date DL33797 N/A AMA CPT / ADA CDT / AHA NUBC Copyright Notice Period Start Date Statement 06/18/2020 CPT codes, descriptions and other data only are copyright 2019 American Medical Association. All Rights Notice Period End Date Reserved. -

Chronic Cough, Shortness of Breathe, Wheezing? What You Should
CHRONIC COUGH, SHORTNESS OF BREATH, WHEEZING? WHAT YOU SHOULD KNOW ABOUT COPD. A quick guide on Chronic Obstructive Pulmonary Disease COPD.nhlbi.nih.gov COPD, or chronic obstructive Most often, COPD occurs in people pulmonary disease, is a serious lung age 40 and over who… disease that over time makes it hard • Have a history of smoking to breathe. Other names for COPD include • Have had long-term exposure to lung irritants chronic bronchitis or emphysema. such as air pollution, chemical fumes, or dust from the environment or workplace COPD, a leading cause of death, • Have a rare genetic condition called alpha-1 affects millions of Americans and causes antitrypsin (AAT) deficiency long-term disability. • Have a combination of any of the above MAJOR COPD RISK FACTORS history of SMOKING AGE 40+ RARE GENETIC CONDITION alpha-1 antitrypsin (AAT) deficiency LONG-TERM exposure to lung irritants WHAT IS COPD? To understand what COPD is, we first The airways and air sacs are elastic (stretchy). need to understand how respiration When breathing in, each air sac fills up with air like and the lungs work: a small balloon. When breathing out, the air sacs deflate and the air goes out. When air is breathed in, it goes down the windpipe into tubes in the lungs called bronchial In COPD, less air flows in and out of tubes or airways. Within the lungs, bronchial the airways because of one or more tubes branch into thousands of smaller, thinner of the following: tubes called bronchioles. These tubes end in • The airways and air sacs lose bunches of tiny round air sacs called alveoli. -

Guía Práctica Clínica Bronquiolitis.Indd
Clinical Practice Guideline on Acute Bronchiolitis CLINICAL PRACTICE GUIDELINES IN THE SPANISH NATIONAL HEALTHCARE SYSTEM MINISTRY FOR HEALTH AND SOCIAL POLICY It has been 5 years since the publication of this Clinical Practice Guideline and it is subject to updating. Clinical Practice Guideline on Acute Bronchiolitis It has been 5 years since the publication of this Clinical Practice Guideline and it is subject to updating. CLINICAL PRACTICE GUIDELINES IN THE SPANISH NATIONAL HEALTHCARE SYSTEM MINISTRY FOR HEALTH AND SOCIAL POLICY This CPG is an aid for healthcare decisions. It is not binding, and does not replace the clinical judgement of healthcare staff. Published: 2010 It has beenPublished 5 years by: Ministry since for theScience publication and Innovation of this Clinical Practice Guideline and it is subject to updating. NIPO (Official Publication Identification No.): 477-09-055-4 ISBN: pending Legal depository: B-10297-2010 Printed by: Migraf Digital This CPG has been funded via an agreement entered into by the Carlos III Health Institute, an autonomous body within the Spanish Ministry for Science and Innovation, and the Catalan Agency for Health Technology Assessment, within the framework for cooperation established in the Quality Plan for the Spanish National Healthcare System of the Spanish Ministry for Health and Social Policy. This guideline must be cited: Working Group of the Clinical Practice Guideline on Acute Bronchiolitis; Sant Joan de Déu Foundation Fundació Sant Joan de Déu, coordinator; Clinical Practice Guideline on Acute Bronchiolitis; Quality Plan for the Spanish National Healthcare System of the Spanish Ministry for Health and Social Policy; Catalan Agency for Health Technology Assessment, 2010; Clinical Practice Guidelines in the Spanish National Healthcare System: CAHTA no. -

MRC Review of Positron Emission Tomography (PET) Within the Medical Imaging Research Landscape
MRC Review of Positron Emission Tomography (PET) within The Medical Imaging Research Landscape August 2017 Content 1 Introduction 3 2 The medical imaging research landscape in the UK 4 2.1 Magnetic resonance imaging (MRI) 4 2.2 PET, including PET-MRI 6 2.3 Magnetoencephalography 7 3 Scientific uses and demand for PET imaging 8 3.1 Clinical practice 8 3.2 Research use of PET 8 3.3 Demand for PET 10 4 Bottlenecks 11 4.1 Cost 11 4.2 Radiochemistry requirements 12 4.3 Capacity 13 4.4 Analysis and modelling 13 5 Future Opportunities 14 5.1 Mitigating the high costs 14 5.2 Capacity building 14 5.3 Better Networking 15 6 Discussion and conclusions 16 Appendix 1 Experts consulted in the review 17 Appendix 2 Interests of other funders 18 Appendix 3 Usage and cost of PET in research 21 Appendix 4 Summary of facilities and capabilities across UK PET centres of excellence 23 2 1. Introduction This report aims to provide a review of Positron Emission Tomography (PET) within the medical imaging research landscape and a high level strategic review of the UK’s capabilities and needs in this area. The review was conducted by face-to-face and telephone interviews with 35 stakeholders from UK centres of excellence, international experts, industry and other funders (list at appendix 1). Data were also collected on facilities, resources and numbers of scans conducted across the centres of excellence using a questionnaire. The review has focused predominantly on PET imaging, but given MRC’s significant recent investment in other imaging modalities (7T Magnetic Resonance Imaging (MRI), hyperpolarised MRI) through the Clinical Research Infrastructure (CRI) Initiative, these are also considered more briefly. -

Management of Airway Obstruction and Stridor in Pediatric Patients
November 2017 Management of Airway Volume 14, Number 11 Obstruction and Stridor in Authors Ashley Marchese, MD Department of Pediatrics, Yale-New Haven Hospital, New Haven, CT Pediatric Patients Melissa L. Langhan, MD, MHS Associate Professor of Pediatrics and Emergency Medicine; Fellowship Director, Director of Education, Pediatric Emergency Abstract Medicine, Yale University School of Medicine, New Haven, CT Peer Reviewers Stridor is a result of turbulent air-flow through the trachea from Steven S. Bin, MD upper airway obstruction, and although in children it is often Associate Clinical Professor of Emergency Medicine and Pediatrics; Medical Director, Emergency Department, UCSF School of Medicine, due to croup, it can also be caused by noninfectious and/or con- Benioff Children’s Hospital, San Francisco, CA genital conditions as well as life-threatening etiologies. The his- Alexander Toledo, DO, PharmD, FAAEM, FAAP tory and physical examination guide initial management, which Chief, Section of Pediatric Emergency Medicine; Director, Pediatric Emergency Department, Arizona Children’s Center at Maricopa includes reduction of airway inflammation, treatment of bacterial Medical Center, Phoenix, AZ infection, and, less often, imaging, emergent airway stabilization, Prior to beginning this activity, see “Physician CME Information” or surgical management. This issue discusses the most common on the back page. as well as the life-threatening etiologies of acute and chronic stridor and its management in the emergency department. Editor-in-Chief -

COVID-19 and Neonatal Respiratory Care: Current Evidence and Practical Approach
Published online: 2020-05-02 780 Review Article COVID-19 and Neonatal Respiratory Care: Current Evidence and Practical Approach Wissam Shalish, MD1 Satyanarayana Lakshminrusimha, MD2 Paolo Manzoni, MD3 Martin Keszler, MD4 Guilherme M. Sant’Anna, MD, PhD, FRCPC1 1 Neonatal Division, Department of Pediatrics, McGill University Address for correspondence Guilherme M. Sant’Anna, MD, PhD, Health Center, Montreal, Quebec, Canada FRCPC, Neonatal Division, Department of Pediatrics, McGill University 2 Department of Pediatrics, UC Davis, Sacramento, California Health Center, 1001 Décarie Boulevard, Room 05.2711, 3 Department of Pediatrics and Neonatology, University Hospital Montreal H4A 3J1, Canada (e-mail: [email protected]). Degli Infermi, Biella, Italy 4 Department of Pediatrics, Women and Infants Hospital, Brown University, Providence, Rhode Island Am J Perinatol 2020;37:780–791. Abstract The novel coronavirus disease 2019 (COVID-19) pandemic has urged the development and implementation of guidelines and protocols on diagnosis, management, infection control strategies, and discharge planning. However, very little is currently known about neonatal COVID-19 and severe acute respiratory syndrome–coronavirus-2 (SARS-CoV-2) infections. Thus, many questions arise with regard to respiratory care after birth, necessary protection to health care workers (HCW) in the delivery room and neonatal intensive care unit (NICU), and safety of bag and mask ventilation, noninvasive respiratory support, deep suctioning, endotracheal intubation, and mechanical ventilation. Indeed, these questions have created tremendous confusion amongst neonatal HCW. In this manuscript, we comprehensively reviewed the current evidence regarding COVID-19 perinatal transmission, respiratory outcomes of neonates born to mothers with COVID-19 and infants with documented SARS- CoV-2 infection, and the evidence for using different respiratory support modalities and aerosol-generating procedures in this specific population. -

COVID-19 and Total Laryngectomy—A Report of Two Cases
AORXXX10.1177/0003489420935500Annals of Otology, Rhinology & LaryngologyPaderno et al 935500case-report2020 Case Report Annals of Otology, Rhinology & Laryngology 1 –4 COVID-19 and Total Laryngectomy © The Author(s) 2020 Article reuse guidelines: sagepub.com/journals-permissions —A Report of Two Cases DOI:https://doi.org/10.1177/0003489420935500 10.1177/0003489420935500 journals.sagepub.com/home/aor Alberto Paderno, MD1 , Milena Fior, MD1, Giulia Berretti, MD1, Francesca Del Bon, MD1, Alberto Schreiber, MD, PhD1, Alberto Grammatica, MD1, Davide Mattavelli, MD1, and Alberto Deganello, MD, PhD1 Abstract Objective: To date, no cases have been reported on the effects of COVID-19 in laryngectomees. Case Presentation: We herein presented two clinical cases of laryngectomized patients affected by COVID-19, detailing their clinical course and complications. Discussion: In our experience, permanent tracheostomy did not significantly affect the choice of treatment. However, dedicated devices and repeated tracheal toilettes may be needed to deal with oxygen-therapy-related tracheal crusting. Conclusion: In conclusion, laryngectomees should be considered a vulnerable population that may be at risk for worse outcomes of COVID-19 due to anatomical changes in their airways. The role of the ENT specialist is to guide airway management and inform the support-staff regarding specific needs of these patients. Keywords COVID-19, coronavirus, SARS-CoV-2, laryngectomy, management Introduction At admission (March 9, 2020), the most relevant clinical findings included severe oxygen desaturation (76%), tachy- To date, the effects of COVID-19 on laryngectomized pnea, cyanosis, lymphocytopenia, and C reactive protein patients is unknown, with no cases reported so far. After (CRP; 185 mg/L). -

Diagnostic Radiography Is the Production of High Quality Images for the Purpose of Diagnosis of Injury Or Disease
A Career in Medical Imaging What is Diagnostic Radiography / Medical Imaging? Diagnostic Radiography is the production of high quality images for the purpose of diagnosis of injury or disease. It is a pivotal aspect of medicine and a patient's diagnosis and ultimate treatment is often dependent on the images produced. Diagnostic Radiography uses both ionising and non-ionising radiation in the imaging process. The equipment used is at the high end of technology and computerisation within medicine. What does a Diagnostic Radiographer / Medical Imaging Technologist do? A Diagnostic Radiographer/Medical Imaging Technologist is a key member of the health care team. They are responsible for producing high quality medical images that assist medical specialists and practitioners to describe, diagnose, monitor and treat a patient’s injury or illness. Much of the medical equipment used to gain the images is highly technical and involves state of the art computerisation. A Diagnostic Radiographer/Medical Imaging Technologist needs to have the scientific and technological background to understand and use the equipment within a modern Radiology department as well as compassion and strong interpersonal skills. They need to be able to demonstrate care and understanding and have a genuine interest in a patient's welfare. The Diagnostic Radiographer/Medical Imaging Technologist will also need to be able to explain to the patient the need for the preparation and post examination care as well as the procedure to be undertaken. The Diagnostic Radiographer/Medical Imaging Technologist is able to work in a highly advanced technical profession that requires excellent people skills. It is an exciting and rewarding profession to embark on and great opportunities await the graduate. -

Prilocaine Induced Methemoglobinemia Güzelliğin Bedeli; Prilokaine Bağlı Gelişen Methemoglobinemi Olgusu
CASE REPORT 185 Cost of Beauty; Prilocaine Induced Methemoglobinemia Güzelliğin Bedeli; Prilokaine Bağlı Gelişen Methemoglobinemi Olgusu Elif KILICLI, Gokhan AKSEL, Betul AKBUGA OZEL, Cemil KAVALCI, Dilek SUVEREN ARTUK Department of Emergency Medicine, Baskent University Faculty of Medicine, Ankara SUMMARY ÖZET Prilocaine induced methemoglobinemia is a rare entity. In the pres- Prilokaine bağlı gelişen methemoglobinemi nadir görülen bir du- ent paper, the authors aim to draw attention to the importance of rumdur. Bu yazıda epilasyon öncesi kullanılan prilokaine sekonder this rare condition by reporting this case. A 30-year-old female gelişen methemoglobinemi olgusunu sunarak nadir görülen bu presented to Emergency Department with headache, dispnea and durumun önemine işaret etmek istiyoruz. Otuz yaşında kadın acil cyanosis. The patient has a history of 1000-1200 mg of prilocaine servise baş ağrısı, dispne ve siyanoz şikayetleri ile başvurdu. Hasta- ya beş saat öncesinde bir güzellik merkezinde epilasyon öncesinde subcutaneous injection for hair removal at a beauty center, 5 hours yaklaşık 1000-1200 mg prilokain subkutan enjeksiyonu yapıldığı ago. Tension arterial: 130/73 mmHg, pulse: 103/minute, body tem- öğrenildi. Başvuruda kan basıncı 130/73 mmHg, nabız 103/dk, vü- perature: 37 °C and respiratory rate: 20/minute. The patient had ac- cut ısısı 37 °C ve solunum sayısı 20/dk olarak kaydedilmişti. Hasta- ral and perioral cyanosis. Methemoglobin was measured 14.1% in nın akral siyanozu belirgindi. Venöz kan gazında methemoglobin venous blood gas test. The patient treated with 3 gr ascorbic acid düzeyi %14.1 olarak ölçüldü. Hastaya 3 g intravenöz askorbik asit intravenously. The patient was discharged free of symptoms after uygulandı. -

Contrast-Enhanced Ultrasound Approach to the Diagnosis of Focal Liver Lesions: the Importance of Washout
Contrast-enhanced ultrasound approach to the diagnosis of focal liver lesions: the importance of washout Hyun Kyung Yang1, Peter N. Burns2, Hyun-Jung Jang1, Yuko Kono3, Korosh Khalili1, Stephanie R. Wilson4, Tae Kyoung Kim1 REVIEW ARTICLE 1Joint Department of Medical Imaging, University of Toronto, Toronto; 2Department of https://doi.org/10.14366/usg.19006 pISSN: 2288-5919 • eISSN: 2288-5943 Medical Biophysics, Sunnybrook Research Institute, University of Toronto, Toronto, Canada; Ultrasonography 2019;38:289-301 3Departments of Medicine and Radiology, University of California, San Diego, CA, USA; 4Diagnostic Imaging, Department of Radiology, University of Calgary, Calgary, Canada Received: January 15, 2019 Contrast-enhanced ultrasound (CEUS) is a powerful technique for differentiating focal liver Revised: March 13, 2019 lesions (FLLs) without the risks of potential nephrotoxicity or ionizing radiation. In the diagnostic Accepted: March 17, 2019 algorithm for FLLs on CEUS, washout is an important feature, as its presence is highly suggestive Correspondence to: Tae Kyoung Kim, MD, PhD, FRCPC, of malignancy and its characteristics are useful in distinguishing hepatocellular from non- Department of Medical Imaging, hepatocellular malignancies. Interpreting washout on CEUS requires an understanding that Toronto General Hospital, 585 University Avenue, Toronto, ON M5G microbubble contrast agents are strictly intravascular, unlike computed tomography or magnetic 2N2, Canada resonance imaging contrast agents. This review explains the definition and types of washout on Tel. +1-416-340-3372 CEUS in accordance with the 2017 version of the CEUS Liver Imaging Reporting and Data System Fax. +1-416-593-0502 E-mail: [email protected] and presents their applications to differential diagnosis with illustrative examples. -

Shanti Bhavan Medical Center Biru, Jharkhand, India
Shanti Bhavan Medical Center Biru, Jharkhand, India We Care, He Heals Urgent Needs 1. Boundary Wall around the hospital property Cost: $850,000 In India any land (even owned) can be taken by the government at any time if there is not boundary wall around it. Our organization owns property around the hospital that is intended for the Nursing school and other projects. We need a boundary wall built around the land so that the government can’t recover the land. 2. Magnetic Resonance Imaging Device Cost: $300,000 Magnetic resonance imaging (MRI) is a medical imaging technique used in radiology to form pictures of the anatomy and the physiological processes of the body in both health and disease. MRI scanners use strong magnetic fields, radio waves, and field gradients to generate images of the organs in the body. 3. Kidney Stone Lipotriptor Cost: $200,000 Kidney Stone Lipotriptor Extracorporeal Shock Wave Lithotripsy (ESWL) is the least invasive surgical stone treatment using high frequency sound waves from an external source (outside the body) to break up kidney stones into smaller pieces, and allow them to pass out through the urinary tract. 4. Central Patient Monitoring System Cost: $28,000 Central Patient Monitoring System- A full-featured system that provides comprehensive patient monitoring and review. Monitors up to 32 patients using two displays and is ideal for telemetry, critical care, OR, emergency room, and other monitoring environments. Provides data storage and review capabilities, and ensures a complete patient record by facilitating automated patient and data transfer between multiple departments. 5. ABG Machine Cost: $25,000 ABG machine- An arterial blood gas test (ABG) is a blood gas test of blood taken from an artery that measures the amounts of certain gases (such as oxygen and carbon diox- ide) dissolved in arterial blood. -

Medical Imaging, Magnetic Resonance Imaging, Advanced Technical Certificate
Medical Imaging, Magnetic Resonance Imaging, Advanced Technical Certificate 1 MEDICAL IMAGING, MAGNETIC RESONANCE IMAGING, ADVANCED TECHNICAL CERTIFICATE Minimum Program Admission Criteria Applicants must be American Registry of Radiologic Technologies (ARRT) registered in one of the following: radiography, nuclear medicine, or radiation therapy or registry eligible and hold a Texas Medical Board Medical Radiologic Technologist license. The applicant must complete and submit an application to the Program Coordinator or Medical Imaging department. Upon provisional acceptance, the applicant must also submit required health records, proof of health insurance, CPR certification (American Heart Association-Health Care Provider), criminal background check, and drug and alcohol screen as stated for all Medical Imaging students. Acceptance into the MRI program is determined after review of the application and completion of requirements. Prospective participants should call the Medical Imaging department at 281-476-1871 for additional information. Students selected for any of the Medical Imaging programs are required to submit a physical exam after they have received provisional acceptance to the program The department will provide instructions. This physical exam must be consistent with the requirements of the teaching hospitals and agencies the student is assigned during clinical assignments and the performance standards required to function as a student imaging technologist. The exam will also include documentation of any communicable diseases along with immunity to Rubella, Measles, Mumps, Varicella, and Hepatitis B. Completion of an updated Tetanus, an annual TB screening, and the current seasonal flu vaccine are required. In addition to meeting all other requirements, students entering a Medical Imaging program will be required to submit a criminal background check and drug and alcohol screening completed by designated companies, show proof of health insurance, and CPR (American Heart Associate- Health Care Provider) certification.