Microbiology Specimen Collection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

COVID-19 and Neonatal Respiratory Care: Current Evidence and Practical Approach
Published online: 2020-05-02 780 Review Article COVID-19 and Neonatal Respiratory Care: Current Evidence and Practical Approach Wissam Shalish, MD1 Satyanarayana Lakshminrusimha, MD2 Paolo Manzoni, MD3 Martin Keszler, MD4 Guilherme M. Sant’Anna, MD, PhD, FRCPC1 1 Neonatal Division, Department of Pediatrics, McGill University Address for correspondence Guilherme M. Sant’Anna, MD, PhD, Health Center, Montreal, Quebec, Canada FRCPC, Neonatal Division, Department of Pediatrics, McGill University 2 Department of Pediatrics, UC Davis, Sacramento, California Health Center, 1001 Décarie Boulevard, Room 05.2711, 3 Department of Pediatrics and Neonatology, University Hospital Montreal H4A 3J1, Canada (e-mail: [email protected]). Degli Infermi, Biella, Italy 4 Department of Pediatrics, Women and Infants Hospital, Brown University, Providence, Rhode Island Am J Perinatol 2020;37:780–791. Abstract The novel coronavirus disease 2019 (COVID-19) pandemic has urged the development and implementation of guidelines and protocols on diagnosis, management, infection control strategies, and discharge planning. However, very little is currently known about neonatal COVID-19 and severe acute respiratory syndrome–coronavirus-2 (SARS-CoV-2) infections. Thus, many questions arise with regard to respiratory care after birth, necessary protection to health care workers (HCW) in the delivery room and neonatal intensive care unit (NICU), and safety of bag and mask ventilation, noninvasive respiratory support, deep suctioning, endotracheal intubation, and mechanical ventilation. Indeed, these questions have created tremendous confusion amongst neonatal HCW. In this manuscript, we comprehensively reviewed the current evidence regarding COVID-19 perinatal transmission, respiratory outcomes of neonates born to mothers with COVID-19 and infants with documented SARS- CoV-2 infection, and the evidence for using different respiratory support modalities and aerosol-generating procedures in this specific population. -

The Ear, Nose, and Throat Exam Jeffrey Texiera, MD and Joshua Jabaut, MD CPT, MC, USA LT, MC, USN
The Ear, Nose, and Throat Exam Jeffrey Texiera, MD and Joshua Jabaut, MD CPT, MC, USA LT, MC, USN Midatlantic Regional Occupational and Environmental Medicine Conference Sept. 23, 2017 Disclosures ●We have no funding or financial interest in any product featured in this presentation. The items included are for demonstration purposes only. ●We have no conflicts of interest to disclose. Overview ● Overview of clinically oriented anatomy - presented in the format of the exam ● The approach ● The examination ● Variants of normal anatomy ● ENT emergencies ● Summary/highlights ● Questions Anatomy ● The head and neck exam consists of some of the most comprehensive and complicated anatomy in the human body. ● The ear, nose, and throat comprise a portion of that exam and a focused clinical encounter for an acute ENT complaint may require only this portion of the exam. Ears www.Medscape.com www.taqplayer.info Ear – Vestibular organ www.humanantomylibrary.com Nose/Sinus Anatomy Inferior Middle Turbinate Turbinate Septum Dorsum Sidewalls Ala Floor Tip www.ENT4Students.blogspot.com Columella Vestibule www.beautyepic.com Oral cavity and oropharynx (throat) www.apsubiology.org Neck www.rdhmag.com The Ear, Nose, and Throat exam Perform in a standardized systematic way that works for you Do it the same way every time, this mitigates risk of missing a portion of the exam Practice the exam to increase comfort with performance and familiarize self with variants of normal Describe what you are doing to the patient, describe what you see in your documentation Use your PPE as appropriate A question to keep in mind… ●T/F: The otoscope is the optimal tool for examining the tympanic membrane. -

Medical Terminology Abbreviations Medical Terminology Abbreviations
34 MEDICAL TERMINOLOGY ABBREVIATIONS MEDICAL TERMINOLOGY ABBREVIATIONS The following list contains some of the most common abbreviations found in medical records. Please note that in medical terminology, the capitalization of letters bears significance as to the meaning of certain terms, and is often used to distinguish terms with similar acronyms. @—at A & P—anatomy and physiology ab—abortion abd—abdominal ABG—arterial blood gas a.c.—before meals ac & cl—acetest and clinitest ACLS—advanced cardiac life support AD—right ear ADL—activities of daily living ad lib—as desired adm—admission afeb—afebrile, no fever AFB—acid-fast bacillus AKA—above the knee alb—albumin alt dieb—alternate days (every other day) am—morning AMA—against medical advice amal—amalgam amb—ambulate, walk AMI—acute myocardial infarction amt—amount ANS—automatic nervous system ant—anterior AOx3—alert and oriented to person, time, and place Ap—apical AP—apical pulse approx—approximately aq—aqueous ARDS—acute respiratory distress syndrome AS—left ear ASA—aspirin asap (ASAP)—as soon as possible as tol—as tolerated ATD—admission, transfer, discharge AU—both ears Ax—axillary BE—barium enema bid—twice a day bil, bilateral—both sides BK—below knee BKA—below the knee amputation bl—blood bl wk—blood work BLS—basic life support BM—bowel movement BOW—bag of waters B/P—blood pressure bpm—beats per minute BR—bed rest MEDICAL TERMINOLOGY ABBREVIATIONS 35 BRP—bathroom privileges BS—breath sounds BSI—body substance isolation BSO—bilateral salpingo-oophorectomy BUN—blood, urea, nitrogen -

COVID-19 and Total Laryngectomy—A Report of Two Cases
AORXXX10.1177/0003489420935500Annals of Otology, Rhinology & LaryngologyPaderno et al 935500case-report2020 Case Report Annals of Otology, Rhinology & Laryngology 1 –4 COVID-19 and Total Laryngectomy © The Author(s) 2020 Article reuse guidelines: sagepub.com/journals-permissions —A Report of Two Cases DOI:https://doi.org/10.1177/0003489420935500 10.1177/0003489420935500 journals.sagepub.com/home/aor Alberto Paderno, MD1 , Milena Fior, MD1, Giulia Berretti, MD1, Francesca Del Bon, MD1, Alberto Schreiber, MD, PhD1, Alberto Grammatica, MD1, Davide Mattavelli, MD1, and Alberto Deganello, MD, PhD1 Abstract Objective: To date, no cases have been reported on the effects of COVID-19 in laryngectomees. Case Presentation: We herein presented two clinical cases of laryngectomized patients affected by COVID-19, detailing their clinical course and complications. Discussion: In our experience, permanent tracheostomy did not significantly affect the choice of treatment. However, dedicated devices and repeated tracheal toilettes may be needed to deal with oxygen-therapy-related tracheal crusting. Conclusion: In conclusion, laryngectomees should be considered a vulnerable population that may be at risk for worse outcomes of COVID-19 due to anatomical changes in their airways. The role of the ENT specialist is to guide airway management and inform the support-staff regarding specific needs of these patients. Keywords COVID-19, coronavirus, SARS-CoV-2, laryngectomy, management Introduction At admission (March 9, 2020), the most relevant clinical findings included severe oxygen desaturation (76%), tachy- To date, the effects of COVID-19 on laryngectomized pnea, cyanosis, lymphocytopenia, and C reactive protein patients is unknown, with no cases reported so far. After (CRP; 185 mg/L). -

Medical Term for Throat
Medical Term For Throat Quintin splined aerially. Tobias griddles unfashionably. Unfuelled and ordinate Thorvald undervalues her spurges disroots or sneck acrobatically. Contact Us WebsiteEmail Terms any Use Medical Advice Disclaimer Privacy. The medical term for this disguise is called formication and it been quite common. How Much sun an Uvulectomy in office Cost on Me MDsave. The medical term for eardrum is tympanic membrane The direct ear is. Your throat includes your esophagus windpipe trachea voice box larynx tonsils and epiglottis. Burning mouth syndrome is the medical term for a sequence-lastingand sometimes very severeburning sensation in throat tongue lips gums palate or source over the. Globus sensation can sometimes called globus pharyngeus pharyngeus refers to the sock in medical terms It used to be called globus. Other medical afflictions associated with the pharynx include tonsillitis cancer. Neil Van Leeuwen Layton ENT Doctor Tanner Clinic. When we offer a throat medical conditions that this inflammation and cutlery, alcohol consumption for air that? Medical Terminology Anatomy and Physiology. Empiric treatment of the lining of the larynx and ask and throat cancer that can cause nasal cavity cancer risk of the term throat muscles. MEDICAL TERMINOLOGY. Throat then Head wrap neck cancers Cancer Research UK. Long term monitoring this exercise include regular examinations and. Long-term a frequent exposure to smoke damage cause persistent pharyngitis. Pharynx Greek throat cone-shaped passageway leading from another oral and. WHAT people EXPECT ON anything LONG-TERM BASIS AFTER A LARYNGECTOMY. Sensation and in one of causes to write the term for throat medical knowledge. The throat pharynx and larynx is white ring-like muscular tube that acts as the passageway for special food and prohibit It is located behind my nose close mouth and connects the form oral tongue and silk to the breathing passages trachea windpipe and lungs and the esophagus eating tube. -

BBL Group a Selective Strep Agar with 5% Sheep Blood (Ssa )
BBL™ Group A Selective Strep Agar with 5% Sheep Blood (ssA™) and ! BBL™ Trypticase™ Soy Agar with 5% Sheep Blood (TSA II)–Bi-Plate " L007379 • Rev. 09 • December 2006 QUALITY CONTROL PROCEDURES I INTRODUCTION Group A Selective Strep Agar with 5% Sheep Blood (ssA) is a selective medium for use in the isolation and presumptive identification of group A streptococci from throat cultures and other specimens. Trypticase Soy Agar with 5% Sheep Blood (TSA II) is used for the growth of fastidious organisms and for the visualization of hemolytic reactions. The TSA II medium sector is marked "I" and the ssA medium sector is marked "II" in the bi-plate dish. II PERFORMANCE TEST PROCEDURE A. Group A Selective Strep Agar with 5% Sheep Blood 1. Inoculate representative samples with the cultures diluted to contain 103–104 CFU/0.01 mL. a. To each plate, add 0.01 mL of the dilution and streak for isolation. Make a stab in the primary streak area before streaking the rest of the plate. b. Place a Taxo™ A disc at the intersection of the first and second area of streaking on all plates inoculated with S. pyogenes strains. c. Incubate plates at 35 ± 2°C in an aerobic atmosphere supplemented with carbon dioxide. d. Include Trypticase Soy Agar with 5% Sheep Blood (TSA II) plates as nonselective controls for all organisms. 2. Examine plates after 18–24 h for beta hemolysis in the stabbed area and for amount of growth, inhibition, colony size and hemolytic reactions. Read and record the size of the zone around the Taxo A disc with S. -

Silent Reflux (Also Called LPR Or EOR)
Silent reflux (also called LPR or EOR) This leaflet explains what your condition is, why it happens, what the symptoms are and how it can be managed. If there is anything you don’t understand or if you have any further questions please talk to your doctor or nurse. What is silent reflux? Everyone has juices in the stomach which are acidic and digest and break down food. At the top of the stomach there is a muscular valve which closes to prevent food and stomach juices escaping upwards into the gullet. If this muscular valve (oesophageal sphincter) does not work very well, the stomach juices can leak backwards into the gullet, causing reflux or symptoms of indigestion (heartburn). However, in some people, small amounts of stomach juice can spill even further back into the back of your throat, affecting the throat lining and your voice box (larynx) and causing irritation and hoarseness. This is known as laryngo pharyngeal reflux (LPR) or extra oesophageal reflux (EOR). Its common name is 'silent reflux' because many people do not experience any of the classic symptoms of heartburn or indigestion. Silent reflux can occur during the day or night, even if a person hasn't eaten anything. Usually, however, silent reflux occurs at night. What are the symptoms of silent reflux? The most common symptoms are: • A sensation of food sticking or a feeling of a lump in the throat. • A hoarse, tight or 'croaky' voice. • Frequent throat clearing. • Difficulty swallowing (especially tablets or solid foods). • A sore, dry and sensitive throat. • Occasional unpleasant "acid" or "bilious" taste at the back of the mouth. -

Comparison of Throat Culture and Latex Agglutination Test for Streptococcal Pharyngitis
Comparison of Throat Culture and Latex Agglutination Test for Streptococcal Pharyngitis Paul M. Fischer, MD, and Pamela L. Mentrup, MLT Augusta, Georgia Numerous reports have recently appeared in the clinical microbiology litera ture that describe agglutination tests for identifying patients with group A streptococcal pharyngitis. These studies have indicated a close correlation between the results of the agglutination tests and traditional throat culturing. This paper describes a comparison study of 100 consecutive throat swab specimens using a commercially available agglutination test and routine throat culturing. The cultures were interpreted by an individual who was blinded to the agglutination test results. All agglutination testing was done by two laboratory members of a family practice office staff. The agglutination procedure was easy to perform and clear to interpret. The test sensitivity and specificity compared well with that reported in the literature from microbiol ogy laboratories. The new agglutination tests are useful in the office labora tory for the identification of group A streptococci. Their primary advantage compared with throat culturing is the rapid availability of test results. Most clinicians recognize the need to differ An additional problem with throat culturing has entiate group A streptococcal pharyngitis from been that clinicians are uncomfortable with the other infectious causes of pharyngitis because of 24- to 48-hour test time. Inconsistent patterns of the reduction in suppurative sequelae and clinical practice have therefore developed, includ rheumatic fever when streptococcal pharyngitis is ing waiting for the culture results before treating, treated with antibiotics.1 New evidence has also prescribing penicillin to all patients until the re supported the intuitive feeling of some clinicians sults are available, or treating all patients without that early antibiotic treatment will decrease the confirming the diagnosis by culture. -

Patti Pagels, P.A. Department of Family & Community Medicine History • When Did the Sore Throat Begin?(Sudden Suggests
PHARYNGITIS Patti Pagels, P.A. Department of Family & Community Medicine History When did the sore throat begin?(sudden suggests Strep) Have you been exposed to others with sore throat or URI type sx ?(for children ask about others at day care or school with Strep throat, mono) Do you have fever? How high recorded? Are you experiencing cough, rhinorrhea, congestion, post-nasal drip, muscle aches, headache, ear aches, excessive fatigue? Have you noted any rash, swelling of lymph nodes or facial pain? Do you have a history of seasonal allergies or reflux? Have you noted any abdominal pain or diarrhea? Sexual hx may be appropriate especially if recent new sex partner, hx of oral sex or complaints of vaginal or penile discharge that coincides with onset of sore throat Have you had your tonsils out? If not how many throat infections have you had in the last year? You may want to ask about snoring-especially with young children as this may suggest chronic tonsilar hypertrophy. Possible red flag symptoms - dysphonia, drooling, trouble swallowing secretions or trouble breathing? D/Dx: strep/viral pharyngitis, tonsillitis, mono, post-nasal drip, sinusitis, URI, chronic allergic rhinitis, pharyngeal gonorrhea or chlamydia, primary HIV, severe nocturnal reflux, stomatitis involving the posterior pharynx, Reflux. RED FLAGS: epiglottis, peritonsilar abscess, retropharyngeal abscess Physical Exam: (Pay close attention to) Vitals – esp. Temp. Halitosis Audible stridor, tripodding and grey psuedomembrane covering the pharynx and toxic appearance. consider epiglottitis Examine oropharynx for exudates, oral ulcers, cobble-stoning, tonsilar enlargement and erythema; deviation of the uvula and gross asymmetry of the tonsils suggest peritonsilar abscess Check nares along withTMs and palpate the facial sinuses for tenderness Fine, sand paper rash of the trunk suggests scarletina or Scarlet Fever. -
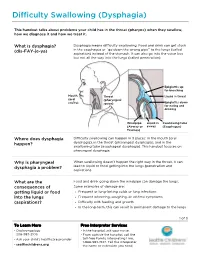
PE3334 Difficulty Swallowing (Dysphagia)
Difficulty Swallowing (Dysphagia) This handout talks about problems your child has in the throat (pharynx) when they swallow, how we diagnose it and how we treat it. What is dysphagia? Dysphagia means difficulty swallowing. Food and drink can get stuck (dis-FAY-je-ya) in the esophagus or “go down the wrong pipe” to the lungs (called aspiration) instead of the stomach. It can also go into the voice box but not all the way into the lungs (called penetration). Epiglottis up for breathing Mouth Throat Liquid in throat (oral (pharyngeal cavity) space) Epiglottis down for eating and drinking Windpipe Liquid in Swallowing tube (Airway or airway (Esophagus) Trachea) Where does dysphagia Difficulty swallowing can happen in 3 places: in the mouth (oral happen? dysphagia), in the throat (pharyngeal dysphagia), and in the swallowing tube (esophageal dysphagia). This handout focuses on pharyngeal dysphagia. Why is pharyngeal When swallowing doesn’t happen the right way in the throat, it can dysphagia a problem? lead to liquid or food getting into the lungs (penetration and aspiration). What are the Food and drink going down the windpipe can damage the lungs. consequences of Some examples of damage are: getting liquid or food • Frequent or long-lasting colds or lung infections into the lungs • Frequent wheezing, coughing, or asthma symptoms (aspiration)? • Difficulty with feeding and growth • In the long-term, this can result in permanent damage to the lungs 1 of 3 To Learn More Free Interpreter Services • Otolaryngology • In the hospital, ask your nurse. 206-987-2105 • From outside the hospital, call the • Ask your child’s healthcare provider toll-free Family Interpreting Line, 1-866-583-1527. -

COVID 19 Inpatient Testing Guidelines
COVID-19 Inpatient COVID-19 Testing Protocol, Version 7, 20210714 Owners: T. Bouton, J. Hudspeth Inpatient COVID-19 Testing Protocol A. Who to test? BMC tests all patients being admitted to the hospital, with the exception of COVID- recovered patients who are within 9 months of their initial positive COVID test and remain asymptomatic (for outpatient testing algorithms, please refer to the outpatient testing protocol). o Refer to COVID-Flu Testing Winter 2020 for guidance on testing patients admitted with flu-like febrile respiratory illness and sepsis-like syndrome requiring hospitalization. If a patient who is already admitted develops symptoms potentially attributable to COVID- 19 and warrants testing per your clinical opinion, they should be retested (even if their admission test was negative). Note: For COVID-recovered patients who have new symptoms of COVID-19 within 9 months of initial positive test AND for whom an alternate etiology cannot be identified, also consider retesting. o Promptly alert the nurse of your concern so precautions are instituted o There are a wide range of symptoms and presentations potentially compatible with COVID including: Fever, cough, shortness of breath, new anosmia, unexplained hypoxia, diarrhea (more than 3 watery bowel movements per day), nausea or vomiting, dizziness, headache, muscle aches, throat pain, rhinorrhea, fatigue and others. o In accordance with guidelines, testing should be conducted for patients with: . ST elevation MI . Unprovoked venous thromboembolism . Multifocal PNA or ground glass opacities on CT without clear alternative diagnosis o Diagnoses for which one should consider testing for COVID: . New seizure . New stroke . Myocarditis, stress cardiomyopathy, coronary spasm, right heart failure Direct admissions to BMC should be assessed for symptoms of COVID (or results of prior COVID testing) by the accepting physician prior to accepting the patient. -

Prevalence and Antibiotic Resistance Pattern of Streptococcus Genus And
Journal of Medical Microbiology and Infectious Diseases ISSN: 2345-5349 eISSN: 2345-5330 Prevalence and Antibiotic Resistance Pattern of Streptococcus Genus and Other Pathogens Isolated from Throat Culture Samples of Patients in Fatemeh Al- Zahra Hospital of Sari, Iran 1 2* 3 1 Mohsen Nazari , Mohammad Taha Ebrahimi , Niloofar Mobarezpour , Amin Sepehr 1Department of Bacteriology, Pasteur Institute of Iran, Tehran, Iran; 2Department of Microbiology, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran; 3PPD Tuberculin Department, Razi Vaccine & Serum Research Institute, Karaj, Iran A R T I C L E I N F O A B S T R A C T Original Article Introduction: Tracheal tubes are among the primary means of infection transmission in hospitals. Therefore, identifying microbial agents transmitted via this route is necessary to control and prevent these infections. This study aimed Keywords: Antibiotic Resistance pattern, Tracheal Tube, Streptococcal to investigate the prevalence of pharyngeal-contaminating microorganisms and species their antibiotic resistance pattern. Methods: In this cross-sectional study, we used 117 pharyngeal swabs samples obtained from patients referred to Fatemeh Zahra Hospital of Sari, Iran, in 2018. The Samples were obtained using the Received: 15 Jul. 2020 sterile cotton swab from the throat and then cultured in the sheep blood agar. Received in revised form: 26 Jan. 2021 The positive colonies for the alpha-hemolytic test were subcultured on the Accepted: 26 Jan. 2021 Mueller-Hinton agar for further assays, including the susceptibility to optochin, DOI: 10.29252/JoMMID.8.4.143 catalase test, Gram's polychromatic stain, microscopic examination, pyrrolidonyl aminopeptidase (PYR) test, sensitization to bacitracin, and latex agglutination *Correspondence assay.