Iron Oxides Aapppplliiccaattiioonnss Nnoottee
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
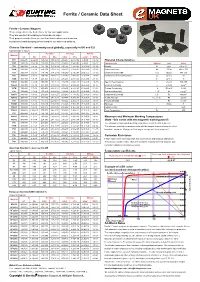
Ferrite / Ceramic Data Sheet
Ferrite / Ceramic Data Sheet Ferrite / Ceramic Magnets These magnets are the best choice for low cost applications. They are excellent at resisting corrosion due to water. Their properties make them an excellent choice when used in motors, loudspeakers and clamping devices and for use with reed switches. Chinese Standard - commonly used globally, especially in UK and EU Typical Range of Values Br Hc (Hcb) Hci (Hcj) BHmax Material mT kG kA/m kOe kA/m kOe kJ/m3 MGOe Y8T 200-235 2.0-2.35 125-160 1.57-2.01 210-280 2.64-3.52 6.5-9.5 0.8-1.2 Physical Characteristics Y10T 200-235 2.0-2.35 128-160 1.61-2.01 210-280 2.64-3.52 6.4-9.6 0.8-1.2 Characteristic Symbol Unit Value Y20 320-380 3.2-3.8 135-190 1.70-2.39 140-195 1.76-2.45 18.0-22.0 2.3-2.8 Density D g/cc 4.9 to 5.1 Y22H 310-360 3.1-3.6 220-250 2.76-3.14 280-320 3.52-4.02 20.0-24.0 2.5-3.0 Vickers Hardness Hv D.P.N 400 to 700 Y23 320-370 3.2-3.7 170-190 2.14-2.39 190-230 2.39-2.89 20.0-25.5 2.5-3.2 Compression Strength C.S N/mm2 680-720 Y25 360-400 3.6-4.0 135-170 1.70-2.14 140-200 1.76-2.51 22.5-28.0 2.8-3.5 Coefficient of Thermal Expansion C// 10-6/C 15 Y26H 360-390 3.6-3.9 220-250 2.76-3.14 225-255 2.83-3.20 23.0-28.0 2.9-3.5 C^ 10-6/C 10 Y26H-1 360-390 3.6-3.9 200-250 2.51-3.14 225-255 2.83-3.20 23.0-28.0 2.9-3.5 Specific Heat Capacity c J/kg°C 795-855 Y26H-2 360-380 3.6-3.8 263-288 3.30-3.62 318-350 4.00-4.40 24.0-28.0 3.0-3.5 Electrical Resistivity r m Ω.cm 1x1010 Y27H 370-400 3.7-4.0 205-250 2.58-3.14 210-255 2.64-3.20 25.0-29.0 3.1-3.6 Thermal Conductivity k W/cm°C 0.029 Y28 370-400 3.7-4.0 175-210 2.20-2.64 180-220 2.26-2.76 26.0-30.0 3.3-3.8 Modulus of Elasticity l / E Pa 1.8x1011 Y28H-1 380-400 3.8-4.0 240-260 3.02-3.27 250-280 3.14-3.52 27.0-30.0 3.4-3.8 Compression Strength C.S. -

Iron (III) Oxide Anhydrous
Material Safety Data Sheet Iron (III) Oxide Anhydrous MSDS# 11521 Section 1 - Chemical Product and Company Identification MSDS Name: Iron (III) Oxide Anhydrous Catalog Numbers: I116-3, I116-500 Synonyms: Ferric Oxide Red; Iron (III) Oxide; Iron Sesquioxide; Red Iron Oxide. Fisher Scientific Company Identification: One Reagent Lane Fair Lawn, NJ 07410 For information in the US, call: 201-796-7100 Emergency Number US: 201-796-7100 CHEMTREC Phone Number, US: 800-424-9300 Section 2 - Composition, Information on Ingredients ---------------------------------------- CAS#: 1309-37-1 Chemical Name: Iron (III) Oxide %: 100 EINECS#: 215-168-2 ---------------------------------------- Hazard Symbols: None listed Risk Phrases: None listed Section 3 - Hazards Identification EMERGENCY OVERVIEW Warning! May cause respiratory tract irritation. May cause mechanical eye and skin irritation. Inhalation of fumes may cause metal-fume fever. Causes severe digestive tract irritation with pain, nausea, vomiting and diarrhea. May corrode the digestive tract with hemorrhaging and possible shock. Target Organs: None. Potential Health Effects Eye: Dust may cause mechanical irritation. Skin: Dust may cause mechanical irritation. May cause severe and permanent damage to the digestive tract. May cause liver damage. Causes severe pain, Ingestion: nausea, vomiting, diarrhea, and shock. May cause hemorrhaging of the digestive tract. The toxicological properties of this substance have not been fully investigated. Dust is irritating to the respiratory tract. Inhalation of fumes may cause metal fume fever, which is characterized Inhalation: by flu-like symptoms with metallic taste, fever, chills, cough, weakness, chest pain, muscle pain and increased white blood cell count. Chronic: Chronic inhalation may cause effects similar to those of acute inhalation. -

A TWO DIMENSIONAL FERRITE-CORE MEMORY Bv M
A TWO DIMENSIONAL FERRITE-CORE MEMORY Bv M. M. FAROOQUI, S. P. SKiVASTAVA AND R. N. NEOGI (Tata Institute of Fundamental Research, Bomba),) Received January t0, 1957 (Communicated by Prof. Bernard P ABS'IXACT This paper describes a two dimensional matrix memory using ferrite- cores. A urtit consisting of 100 words of I 1 binary digits each has been cortstructed for parallel operation. The word drive is from a biasr switch-core matrix, while the digit drive makes use of pulse--trans- formers. Logic and circuit techniques enable high discrimination bet- ween wanted and unwanted signals. A semi-automatic method for testing the memory cores is also described. INTRODUCTION THE potentiality of magnetie fer¡ with a rectangular hysteresis loop as binary storage elements for digital computer was realised earlier by Forrester. x Later, Papian% s and almost simultaneously Rajchman4, ~ showed the practicability of such a system by successfully operating memories of fairly large capacity. This paper describes a modest attempt in this direction. A memory of I00 words, 11 bits each, has beca constructed to operate with a smaU digital computer at the Tata institute of Fundamental Research, Bombay. THE PRINClPLE OF OPERATION OF THE MEMORY The two stable states required for sto¡ binary information in magnetic cores ate the states of positive and negative magnetisation. These are the states corresponding to the position A0 and Ax on the hysteresis loop and can be termed as the 0- and the 1-states respectively (Fig. 2). Con- sider a number of sucia cores with ah ideaUy rectangular hysteresis character- istic, arranged in rows and columns in the form of a matrix. -

Depositional Setting of Algoma-Type Banded Iron Formation Blandine Gourcerol, P Thurston, D Kontak, O Côté-Mantha, J Biczok
Depositional Setting of Algoma-type Banded Iron Formation Blandine Gourcerol, P Thurston, D Kontak, O Côté-Mantha, J Biczok To cite this version: Blandine Gourcerol, P Thurston, D Kontak, O Côté-Mantha, J Biczok. Depositional Setting of Algoma-type Banded Iron Formation. Precambrian Research, Elsevier, 2016. hal-02283951 HAL Id: hal-02283951 https://hal-brgm.archives-ouvertes.fr/hal-02283951 Submitted on 11 Sep 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Accepted Manuscript Depositional Setting of Algoma-type Banded Iron Formation B. Gourcerol, P.C. Thurston, D.J. Kontak, O. Côté-Mantha, J. Biczok PII: S0301-9268(16)30108-5 DOI: http://dx.doi.org/10.1016/j.precamres.2016.04.019 Reference: PRECAM 4501 To appear in: Precambrian Research Received Date: 26 September 2015 Revised Date: 21 January 2016 Accepted Date: 30 April 2016 Please cite this article as: B. Gourcerol, P.C. Thurston, D.J. Kontak, O. Côté-Mantha, J. Biczok, Depositional Setting of Algoma-type Banded Iron Formation, Precambrian Research (2016), doi: http://dx.doi.org/10.1016/j.precamres. 2016.04.019 This is a PDF file of an unedited manuscript that has been accepted for publication. -

Earth Systems Science Grades 9-12
Earth Systems Science Grades 9-12 Lesson 2: The Irony of Rust The Earth can be considered a family of four major components; a biosphere, atmosphere, hydrosphere, and geosphere. Together, these interacting and all-encompassing subdivisions constitute the structure and dynamics of the entire Earth. These systems do not, and can not, stand alone. This Module demonstrates, at every grade level, the concept that one system depends on every other for molding the Earth into the world we know. For example, the biosphere could not effi ciently prosper as is without gas exchange from the atmosphere, liquid water from the hydrosphere, and food and other materials provided by the geosphere. Similarly, the other systems are signifi cantly affected by the biosphere in one way or another. This Module uses Earth’s systems to provide the ultimate lesson in teamwork. March 2006 2 JOURNEY THROUGH THE UNIVERSE Lesson 2: The Irony of Rust Lesson at a Glance Lesson Overview In this lesson, students will investigate the chemistry of rust—the forma- tion of iron oxide (Fe2O3)—within a modern context, by experimenting with the conditions under which iron oxide forms. Students will apply what they have learned to deduce the atmospheric chemistry at the time that the sediments, which eventually became common iron ore found in the United States and elsewhere, were deposited. Students will interpret the necessary formation conditions of this iron-bearing rock in the context of Earth’s geochemical history and the history of life on Earth. Lesson Duration Four 45-minute class periods plus 10 minutes a day for maintence and observation for two weeks Core Education Standards National Science Education Standards Standard B3: A large number of important reactions involve the transfer of either electrons (oxidation/reduction reactions) or hydrogen ions (acid/base reactions) between reacting ions, molecules, or atoms. -

Banded Iron Formations
Banded Iron Formations Cover Slide 1 What are Banded Iron Formations (BIFs)? • Large sedimentary structures Kalmina gorge banded iron (Gypsy Denise 2013, Creative Commons) BIFs were deposited in shallow marine troughs or basins. Deposits are tens of km long, several km wide and 150 – 600 m thick. Photo is of Kalmina gorge in the Pilbara (Karijini National Park, Hamersley Ranges) 2 What are Banded Iron Formations (BIFs)? • Large sedimentary structures • Bands of iron rich and iron poor rock Iron rich bands: hematite (Fe2O3), magnetite (Fe3O4), siderite (FeCO3) or pyrite (FeS2). Iron poor bands: chert (fine‐grained quartz) and low iron oxide levels Rock sample from a BIF (Woudloper 2009, Creative Commons 1.0) Iron rich bands are composed of hematitie (Fe2O3), magnetite (Fe3O4), siderite (FeCO3) or pyrite (FeS2). The iron poor bands contain chert (fine‐grained quartz) with lesser amounts of iron oxide. 3 What are Banded Iron Formations (BIFs)? • Large sedimentary structures • Bands of iron rich and iron poor rock • Archaean and Proterozoic in age BIF formation through time (KG Budge 2020, public domain) BIFs were deposited for 2 billion years during the Archaean and Proterozoic. There was another short time of deposition during a Snowball Earth event. 4 Why are BIFs important? • Iron ore exports are Australia’s top earner, worth $61 billion in 2017‐2018 • Iron ore comes from enriched BIF deposits Rio Tinto iron ore shiploader in the Pilbara (C Hargrave, CSIRO Science Image) Australia is consistently the leading iron ore exporter in the world. We have large deposits where the iron‐poor chert bands have been leached away, leaving 40%‐60% iron. -

Magnetics in Switched-Mode Power Supplies Agenda
Magnetics in Switched-Mode Power Supplies Agenda • Block Diagram of a Typical AC-DC Power Supply • Key Magnetic Elements in a Power Supply • Review of Magnetic Concepts • Magnetic Materials • Inductors and Transformers 2 Block Diagram of an AC-DC Power Supply Input AC Rectifier PFC Input Filter Power Trans- Output DC Outputs Stage former Circuits (to loads) 3 Functional Block Diagram Input Filter Rectifier PFC L + Bus G PFC Control + Bus N Return Power StageXfmr Output Circuits + 12 V, 3 A - + Bus + 5 V, 10 A - PWM Control + 3.3 V, 5 A + Bus Return - Mag Amp Reset 4 Transformer Xfmr CR2 L3a + C5 12 V, 3 A CR3 - CR4 L3b + Bus + C6 5 V, 10 A CR5 Q2 - + Bus Return • In forward converters, as in most topologies, the transformer simply transmits energy from primary to secondary, with no intent of energy storage. • Core area must support the flux, and window area must accommodate the current. => Area product. 4 3 ⎛ PO ⎞ 4 AP = Aw Ae = ⎜ ⎟ cm ⎝ K ⋅ΔB ⋅ f ⎠ 5 Output Circuits • Popular configuration for these CR2 L3a voltages---two secondaries, with + From 12 V 12 V, 3 A a lower voltage output derived secondary CR3 C5 - from the 5 V output using a mag CR4 L3b + amp postregulator. From 5 V 5 V, 10 A secondary CR5 C6 - CR6 L4 SR1 + 3.3 V, 5 A CR8 CR7 C7 - Mag Amp Reset • Feedback to primary PWM is usually from the 5 V output, leaving the +12 V output quasi-regulated. 6 Transformer (cont’d) • Note the polarity dots. Xfmr CR2 L3a – Outputs conduct while Q2 is on. -

Combustion of Iron Wool – Student Sheet
Combustion of iron wool – Student sheet To study Iron is a metal. Iron wool is made up of thin strands of iron loosely bundled together. Your teacher has attached a piece of iron wool to a see-saw balance. At the other end of the see-saw is a piece of Plasticine. Iron wool can combust. Your teacher is going to make the iron wool combust by heating it. If there is a change in mass, the see-saw will either tip to the left or to the right. To discuss or to answer 1 What do you think will happen? ............................................................................................................................................................. 2 Why do you think this will happen? ............................................................................................................................................................. ............................................................................................................................................................. 3 What do you see happen when it is demonstrated? ............................................................................................................................................................. 4 Was your prediction correct? ............................................................................................................................................................. Nuffield Practical Work for Learning: Model-based Inquiry • Combustion of iron wool • Student sheets page 1 of 4 © Nuffield Foundation 2013 • downloaded from -

Ferrite and Metal Composite Inductors
Ferrite and Metal Composite Inductors Design and Characteristics © 2019 KEMET Corporation What is an Inductor? Coil Magnetic Magnetic flux φ (Wire) Field dφ Core e = - dt Material i The coil converts electric energy into magnetic energy and stores it. e Core Material Current through the coil of wire creates a magnetic field Air Ferrite Metal and stores it. (None) (Iron) Composite Different core materials change magnetic field strength. © 2019 KEMET Corporation Ferrite Inductor or Metal Composite Inductor? Ferrite InductorFerrite Metal InductorMetal Composite MaterialMaterial Type type Ni-ZnNi-Zn Mn-Zn Mn-Zn Fe based Fe Based Very Good!! No Good.. InductanceInductace GoodGood! Very Good No Good Very Good!! Magnetic Saturation Good! No Good.. Good! No Good.. Very Good!! MagneticThermal Saturation Property Good No Good Very Good Good! Very Good!! Good! Efficiency Very Good!! No Good.. Good! ThermalResistance Property of core Good No Good Very Good Efficiency SBC/SBCPGood TPI Very GoodMPC, MPCV Good Products MPLC, MPLCV Resistance of Core Very Good No Good Good © 2019 KEMET Corporation Ferrite and Metal Composite Comparison Advantage of Ferrite 1. Higher inductance with high permeability 2. Stable inductance in the right range High L and Low DCR capability Advantage of Metal Composite 1. Very slow saturation 2. Very stable saturation for the thermal Core Loss Comparison Good for Auto app especially Advantage of Ferrite Very low core loss in dynamic frequency range Mn-Zn Ferrite Metal Core Low power consumption capability © 2019 KEMET Corporation -

Switch Mode Power Supplies and Their Magnetics Tutorial
Datatronic Switch Mode Power Supplies and their Magnetics Many factors must be considered by designers when choosing the magnetic components required in today’s electronic power supplies DATATRONIC DISTRIBUTION, INC. Datatronic In today’s day and age the most often used topology for electronic power supplies is that of the Switch Mode Power Supply (SMPS), which is a major user of magnetics. In some applications the “older type” linear supplies are still used, but in the early 70’s SMPS came into being spurred by the development of faster switching transistors. This facilitated the use of much smaller magnetic components and greater efficiencies. DATATRONIC DISTRIBUTION, INC. Datatronic SMPS and their General Magnetic Usage In general, there are four different types of magnetic components that are needed for the typical SMPS. They include the Output Transformer, usually the most noticeable because of its size compared to the others, the Output Inductors, the Input Inductors and the Current Sense Transformer, each with its own important function. DATATRONIC DISTRIBUTION, INC. Datatronic SMPS and their General Magnetic Usage 1.The Output Transformer or “Main” Transformer takes the input voltage that is supplied to its primary winding and then transforms the input voltage to one or more voltages that are the output of the secondary winding or windings. 2. The Output Inductors are used to filter the output voltage so that the load “sees” a filtered DC voltage. DATATRONIC DISTRIBUTION, INC. Datatronic SMPS and their General Magnetic Usage 3. The Input Inductors filter out the noise generated by the switching transistors so that this noise isn’t emitted back to the source. -
Iron Oxide Pigments Data Sheet
90 IRON OXIDE PIGMENTS (Data in metric tons unless otherwise noted) Domestic Production and Use: Iron oxide pigments (IOPs) were mined domestically by two companies in two States. Mine production, which was withheld to avoid disclosing company proprietary data, decreased in 2019 from that of 2018. Five companies, including the two producers of natural IOPs, processed and sold about 38,000 tons of finished natural and synthetic IOPs with an estimated value of $52 million, significantly below the most recent sales peak of 88,100 tons in 2007. About 59% of natural and synthetic finished IOPs were used in concrete and other construction materials; 11% in plastics; 7% in coatings and paints; 5% in foundry sands and other foundry uses; 3% each in animal food, industrial chemicals, and glass and ceramics; and 9% in other uses. Salient Statistics—United States: 2015 2016 2017 2018 2019e Mine production, crude W W W W W Sold or used, finished natural and synthetic IOP 53,500 48,500 47,300 48,200 38,000 Imports for consumption 176,000 179,000 179,000 179,000 160,000 Exports, pigment grade 8,930 15,800 13,500 11,100 9,900 Consumption, apparent1 221,000 212,000 213,000 216,000 190,000 Price, average value, dollars per kilogram2 1.46 1.46 1.46 1.58 1.40 Employment, mine and mill 55 60 60 60 55 Net import reliance3 as a percentage of: Apparent consumption W W W W W Reported consumption >50 >50 >50 >50 >50 Recycling: None. Import Sources (2015–18): Natural: Spain, 43%; Cyprus, 36%; Austria, 10%; France, 9%; and other, 2%. -

INTRODUCTION:- Ferrites Are Ferromagnetic Material Containing Predominantly Oxides Iron Along with Other Oxides of Barium, Stron
INTRODUCTION:- Ferrites are ferromagnetic material containing predominantly oxides iron along with other oxides of barium, strontium, manganese, nickel, zinc, lithium and cadmium .Ferrites are ideally suited for making device like inductor core, circulators, memory devices and also for various microwave application. Although the saturation magnetisation of ferrites less than that of ferromagnetic alloys, they have advantages such as applicability at higher frequency, lower price and greater electrical resistance. Since 1950, soft ferrites have been widely studied and have become a field of interest of many researches because of their application potential in the modern electronics industry. The electrical and magnetic properties of these materials are structure sensitive and can be altered by doping or substitution. The substitution of aluminium in the inverse ferrites like Nickel, copper and cobalt ferrites have proved to be useful by increasing their saturation, magnetization, resistivity, however, at the cost of decrease of Curie temperature. Cadmium ferrite is a normal spinel ; its magnetic moment per unit cell is zero. Low magnetic high resistive ferrite are so applicable in the high frequency transformer cores. The addition of aluminium which has strong preference for the octahedral sites should exhibit the decrease of the magnetisation because aluminium is nonmagnetic. As aluminium content increases, the magnetic moment of unit cell decreases that means show triangular spin moment, their occurs a reduction in the sub lattice interaction. The removal of iron ions from magnetic sublattice and substitution of the nonmagnetic aluminium ion in its place weakens the magnetism. Due to this substitution improves catalytic, dielectric and magnetic properties, as they possess high resistivity and negligible eddy current losses.