Kava: a Review of the Safety of Traditional and Recreational Beverage Consumption
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
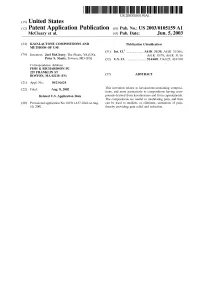
(12) Patent Application Publication (10) Pub. No.: US 2003/0105159 A1 Mccleary Et Al
US 200301 05159A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0105159 A1 McCleary et al. (43) Pub. Date: Jun. 5, 2003 (54) KAVALACTONE COMPOSITIONS AND Publication Classification METHODS OF USE (51) Int. Cl." ....................... A61K 31/35; A61K 31/366; (76) Inventors: Joel McCleary, The Plains, VA (US); A61K 35/78; A61K 31/16 Peter S. Staats, Towson, MD (US) (52) U.S. Cl. ........................... 514/460; 514/625; 424/760 Correspondence Address: FISH & RICHARDSON PC 225 FRANKLIN ST BOSTON, MA 02110 (US) (57) ABSTRACT (21) Appl. No.: 10/214,624 (22) Filed: Aug. 8, 2002 This invention relates tO kavalactone-containing composi tions, and more particularly to compositions having com Related U.S. Application Data pounds derived from kavalactones and from capsaicinoids. The compositions are useful in modulating pain, and thus (60) Provisional application No. 60/311,437, filed on Aug. can be used to mediate, or eliminate, Sensations of pain, 10, 2001. thereby providing pain relief and reduction. US 2003/0105159 A1 Jun. 5, 2003 KAVALACTONE COMPOSITIONS AND METHODS 0006. In one embodiment, the invention relates to an OF USE analgesic topical composition having: (a) a kavalactone; (b) capsaicinoid or Synthetic derivatives thereof; and (c) a CROSS-REFERENCE TO RELATED pharmaceutically acceptable carrier; wherein the weight APPLICATIONS ratio of(a):(b) is from 5000:1 to 1:2 (e.g., 800:1 to 1:1; 500:1 to 5:1). In other aspects, the composition includes an effec 0001. This application claims benefit of U.S. application tive amount of kavalactones, active kavalactones, or capsai Ser. -

Kava (Piper Methysticum) and Its Methysticin Constituents Protect Brain Tissue Against Ischemic Damage in Rodents
5 Refs: Arletti R et al, Stimulating property of Turnera diffusa and Pfaffia paniculata extracts on the sexual-behavior of male rats. Psychopharmacology 143(1), 15-19, 1999. Berger F, Handbuch der drogenkunde . Vol 2, Maudrich, Wien, 1950. Martinez M, Les plantas medicinales de Mexico . Cuarta Edicion Botas Mexico , p119, 1959. Tyler VE et al, Pharmacognosy , 9 th edition, Lea & Febiger, Philadelphia, 1988. KAVA ( Piper methysticum ) - A REVIEW The Kava plant (Piper methysticum) is a robust, well-branching and erect perennial shrub belonging to the pepper family (Piperaceae). The botanical origin remains unknown, although it is likely that early Polynesian explorers brought the plant with them from island to island. Numerous varieties of Kava exist, and today it is widely cultivated in several Pacific Island countries both for local use as well as the rapidly growing demand for pharmaceutical preparations. The dried rhizomes (roots) are normally used. The first description to the western world of the ceremonial use of an intoxicating beverage prepared from Kava was made by Captain James Cook following his Pacific voyage in 1768. The drink, prepared as an infusion in an elaborate manner after first chewing the root, is consumed on formal occasions or meetings of village elders and chiefs, as well as in reconciling with enemies and on a more social basis. It remains an important social custom in many Pacific Island countries today. Most of the islands of the Pacific possessed Kava prior to European contact, particularly those encompassed by Polynesia, Melanesia and Micronesia. After drinking the Kava beverage a pleasantly relaxed and sociable state develops, after which a deep and restful sleep occurs. -

Alternative Treatments for Depression and Anxiety
2019 PCB Conference: Strickland Benzodiazepines (BZDs), Herbal and Alternative Treatments for Anxiety & Depression BZD Learning Objectives • List at least three uses for benzodiazepines • Discuss at least two risk factors associated with benzodiazepine prescriptions Craig Strickland, PhD, Owner Biobehavioral Education and Consultation https://sites.google.com/site/bioedcon 1 2 BZD Pharmacokinetics Clinical Uses of BZDs Generic Name Trade Name Rapidity ½ Life Dose (mg) • Treat a variety of anxiety disorders alprazolam Xanax Intermediate Short 0.75-4 • Hypnotics • Muscle relaxants chlordiaze- Librium Intermediate Long 15-100 poxide • To produce anterograde amnesia clonazepam Klonopin Intermediate Long 0.5-4 • Alcohol & other CNS depressant withdrawal • Anti-convulsant therapy diazepam Valium Rapid Long 4-40 triazolam Halcion Intermediate Very short 0.125-0.5 temazepam Restoril Short Short 7.5-30 3 4 1 2019 PCB Conference: Strickland Issues with BZDs Herbal Medication and Alternative Therapies Used in the Treatment of Depression and Anxiety • Addictive potential • Confusion between “anti-anxiety” effects and the “warm-fuzzy) • Large dose ranges • Comparison of BZDs with medications like Buspar, etc. • They work, they work well and they work quickly 5 6 Alternative Tx. Learning Objectives Background Information on herbals: Natural does not necessarily mean “safe” • List several amino acid treatments for depression • Side-effects and adverse reactions • List at least three of the most common herbal – Herbal medications are “drugs” although -

(12) Patent Application Publication (10) Pub. No.: US 2016/017.4603 A1 Abayarathna Et Al
US 2016O174603A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/017.4603 A1 Abayarathna et al. (43) Pub. Date: Jun. 23, 2016 (54) ELECTRONIC VAPORLIQUID (52) U.S. Cl. COMPOSITION AND METHOD OF USE CPC ................. A24B 15/16 (2013.01); A24B 15/18 (2013.01); A24F 47/002 (2013.01) (71) Applicants: Sahan Abayarathna, Missouri City, TX 57 ABSTRACT (US); Michael Jaehne, Missouri CIty, An(57) e-liquid for use in electronic cigarettes which utilizes- a TX (US) vaporizing base (either propylene glycol, vegetable glycerin, (72) Inventors: Sahan Abayarathna, MissOU1 City,- 0 TX generallyor mixture at of a 0.001 the two) g-2.0 mixed g per with 1 mL an ratio. herbal The powder herbal extract TX(US); (US) Michael Jaehne, Missouri CIty, can be any of the following:- - - Kanna (Sceletium tortuosum), Blue lotus (Nymphaea caerulea), Salvia (Salvia divinorum), Salvia eivinorm, Kratom (Mitragyna speciosa), Celandine (21) Appl. No.: 14/581,179 poppy (Stylophorum diphyllum), Mugwort (Artemisia), Coltsfoot leaf (Tussilago farfara), California poppy (Eschscholzia Californica), Sinicuichi (Heimia Salicifolia), (22) Filed: Dec. 23, 2014 St. John's Wort (Hypericum perforatum), Yerba lenna yesca A rtemisia scoparia), CaleaCal Zacatechichihichi (Calea(Cal termifolia), Leonurus Sibericus (Leonurus Sibiricus), Wild dagga (Leono Publication Classification tis leonurus), Klip dagga (Leonotis nepetifolia), Damiana (Turnera diffiisa), Kava (Piper methysticum), Scotch broom (51) Int. Cl. tops (Cytisus scoparius), Valarien (Valeriana officinalis), A24B 15/16 (2006.01) Indian warrior (Pedicularis densiflora), Wild lettuce (Lactuca A24F 47/00 (2006.01) virosa), Skullcap (Scutellaria lateriflora), Red Clover (Trifo A24B I5/8 (2006.01) lium pretense), and/or combinations therein. -

Could Mycolactone Inspire New Potent Analgesics? Perspectives and Pitfalls
toxins Review Could Mycolactone Inspire New Potent Analgesics? Perspectives and Pitfalls 1 2 3, 4, , Marie-Line Reynaert , Denis Dupoiron , Edouard Yeramian y, Laurent Marsollier * y and 1, , Priscille Brodin * y 1 France Univ. Lille, CNRS, Inserm, CHU Lille, Institut Pasteur de Lille, U1019-UMR8204-CIIL-Center for Infection and Immunity of Lille, F-59000 Lille, France 2 Institut de Cancérologie de l’Ouest Paul Papin, 15 rue André Boquel-49055 Angers, France 3 Unité de Microbiologie Structurale, Institut Pasteur, CNRS, Univ. Paris, F-75015 Paris, France 4 Equipe ATIP AVENIR, CRCINA, INSERM, Univ. Nantes, Univ. Angers, 4 rue Larrey, F-49933 Angers, France * Correspondence: [email protected] (L.M.); [email protected] (P.B.) These three authors contribute equally to this work. y Received: 29 June 2019; Accepted: 3 September 2019; Published: 4 September 2019 Abstract: Pain currently represents the most common symptom for which medical attention is sought by patients. The available treatments have limited effectiveness and significant side-effects. In addition, most often, the duration of analgesia is short. Today, the handling of pain remains a major challenge. One promising alternative for the discovery of novel potent analgesics is to take inspiration from Mother Nature; in this context, the detailed investigation of the intriguing analgesia implemented in Buruli ulcer, an infectious disease caused by the bacterium Mycobacterium ulcerans and characterized by painless ulcerative lesions, seems particularly promising. More precisely, in this disease, the painless skin ulcers are caused by mycolactone, a polyketide lactone exotoxin. In fact, mycolactone exerts a wide range of effects on the host, besides being responsible for analgesia, as it has been shown notably to modulate the immune response or to provoke apoptosis. -

Yangonin Blocks Tumor Necrosis Factor-Α–Induced Nuclear Factor-Κb–Dependent Transcription by Inhibiting the Transactivation Potential of the Rela/P65 Subunit
J Pharmacol Sci 118, 447 – 454 (2012) Journal of Pharmacological Sciences © The Japanese Pharmacological Society Full Paper Yangonin Blocks Tumor Necrosis Factor-α–Induced Nuclear Factor-κB–Dependent Transcription by Inhibiting the Transactivation Potential of the RelA/p65 Subunit Juan Ma1,†, He Liang1,†, Hong Ri Jin2, Nguyen Tien Dat3, Shan Yu Zhang1, Ying Zi Jiang1, Ji Xing Nan1, Donghao Li1, Xue Wu1, Jung Joon Lee1,2,*a, and Xuejun Jin1,*b 1Key Laboratory of Natural Resources of Changbai Mountain & Functional Molecules, Yanbian University, Ministry of Education, Yanji Jilin 133002, China 2Center for Molecular Cancer Research, Korea Research Institute of Bioscience and Biotechnology, Ochang, Chungbuk 363-883, Republic of Korea 3Institute of Marine Biochemistry, Vietnam Academy of Science and Technology, 18-Hoang Quoc Viet, Hanoi, Vietnam Received November 13, 2011; Accepted January 23, 2012 Abstract. The nuclear factor-κB (NF-κB) transcription factors control many physiological pro- cesses including inflammation, immunity, and apoptosis. In our search for NF-κB inhibitors from natural resources, we identified yangonin from Piper methysticum as an inhibitor of NF-κB activa- tion. In the present study, we demonstrate that yangonin potently inhibits NF-κB activation through suppression of the transcriptional activity of the RelA/p65 subunit of NF-κB. This compound sig- nificantly inhibited the induced expression of the NF-κB-reporter gene. However, this compound did not interfere with tumor necrosis factor-α (TNF-α)-induced inhibitor of κBα (IκBα) degrada- tion, p65 nuclear translocation, and DNA-binding activity of NF-κB. Further analysis revealed that yangonin inhibited not only the induced NF-κB activation by overexpression of RelA/p65, but also transactivation activity of RelA/p65. -

(12) United States Patent (10) Patent No.: US 9.421,180 B2 Zielinski Et Al
USOO9421 180B2 (12) United States Patent (10) Patent No.: US 9.421,180 B2 Zielinski et al. (45) Date of Patent: Aug. 23, 2016 (54) ANTIOXIDANT COMPOSITIONS FOR 6,203,817 B1 3/2001 Cormier et al. .............. 424/464 TREATMENT OF INFLAMMATION OR 6,323,232 B1 1 1/2001 Keet al. ............ ... 514,408 6,521,668 B2 2/2003 Anderson et al. ..... 514f679 OXIDATIVE DAMAGE 6,572,882 B1 6/2003 Vercauteren et al. ........ 424/451 6,805,873 B2 10/2004 Gaudout et al. ....... ... 424/401 (71) Applicant: Perio Sciences, LLC, Dallas, TX (US) 7,041,322 B2 5/2006 Gaudout et al. .............. 424/765 7,179,841 B2 2/2007 Zielinski et al. .. ... 514,474 (72) Inventors: Jan Zielinski, Vista, CA (US); Thomas 2003/0069302 A1 4/2003 Zielinski ........ ... 514,452 Russell Moon, Dallas, TX (US); 2004/0037860 A1 2/2004 Maillon ...... ... 424/401 Edward P. Allen, Dallas, TX (US) 2004/0091589 A1 5, 2004 Roy et al. ... 426,265 s s 2004/0224004 A1 1 1/2004 Zielinski ..... ... 424/442 2005/0032882 A1 2/2005 Chen ............................. 514,456 (73) Assignee: Perio Sciences, LLC, Dallas, TX (US) 2005, 0137205 A1 6, 2005 Van Breen ..... 514,252.12 2005. O154054 A1 7/2005 Zielinski et al. ............. 514,474 (*) Notice: Subject to any disclaimer, the term of this 2005/0271692 Al 12/2005 Gervasio-Nugent patent is extended or adjusted under 35 et al. ............................. 424/401 2006/0173065 A1 8/2006 BeZwada ...................... 514,419 U.S.C. 154(b) by 19 days. 2006/O193790 A1 8/2006 Doyle et al. -

Anti-Inflammatory Activity of Compounds from Kaempferia Marginata Rhizomes
Songklanakarin J. Sci. Technol. 39 (1), 91-99, Jan. - Feb. 2017 http://www.sjst.psu.ac.th Original Article Anti-inflammatory activity of compounds from Kaempferia marginata rhizomes Kanidta Kaewkroek1, Chatchai Wattanapiromsakul1, 2, Hisashi Matsuda3, Seikou Nakamura3, and Supinya Tewtrakul1, 2* 1 Department of Pharmacognosy and Pharmaceutical Botany, Faculty of Pharmaceutical Sciences, Prince of Songkla University, Hat Yai, Songkhla, 90112 Thailand 2 Excellent Research Laboratory, Phytomedicine and Phamaceutical Biotechnology Excellence Center, Faculty of Pharmaceutical Sciences, Prince of Songkla University, Hat Yai, Songkhla, 90112 Thailand 3 Kyoto Pharmaceutical University, Misasagi, Yamashina-ku, Kyoto, 607-8412 Japan Received: 12 January 2016; Revised: 12 April 2016; Accepted: 19 April 2016 Abstract Two new pimarane diterpenes were obtained from Kaempferia marginata rhizomes, which are 1-acetoxysandara- copimaradien-2-one (1) and 1-acetoxysandaracopimaradiene (4), along with seven known compounds from the hexane and chloroform fractions including two pimarane-type diterpenes [marginatol (5), sandaracopimaradiene (8)], one kavalactone [desmethoxyyangonin (3)], three steroids [sitosterol--D-glucoside (2), the mixture of stigmasterol and -sitosterol (6 + 7)] and one diarylheptanoid [bisdemethoxycurcumin (9)]. Compounds 3 and 9 exhibited potent effect against NO production with IC50 of 10.1 and 6.8 µM, respectively. Compound 3 inhibited iNOS mRNA expression in a dose-dependent manner, while 9 suppressed both of iNOS and COX-2 genes. Moreover, compounds 2, 3, 6 + 7 and 9 were isolated for the first time from K. marginata. These results revealed that diterpenes, diarylheptanoid and kavalactone are components of K. marginata that afford anti-inflammatory effect through a mechanism involving a decrease in inflammatory mediators. Keywords: Kaempferia marginata, diterpenes, diarylheptanoid, kavalactone, anti-inflammatory activity 1. -

Is Alcohol Use Related to High Cholesterol in Premenopausal
Research iMedPub Journals Journal of Preventive Medicine 2018 http://www.imedpub.com/ Vol.3 No.1:3 ISSN 2572-5483 DOI: 10.21767/2572-5483.100024 Is Alcohol Use Related To High Cholesterol in Premenopausal Women Aged 40-51 Years Old? Sydnee Homeyer, Jessica L Hartos*, Holli Lueg, Jessica Moore and Patricia Stafford Department of Physician Assistant Studies, University of North Texas Health Science Center, USA *Corresponding author: Jessica L. Hartos, Department of Physician Assistant Studies, University of North Texas Health Science Center, USA, Tel: 817-735-2454; Fax: 817-735-2529; E-mail: [email protected] Received date: November 13, 2017; Accepted date: January 8, 2018; Publication date: January 15, 2018 Citation: Homeyer S, Hartos JL, Lueg H, Moore J, Stafford P, et al. (2018) Is Alcohol Use Related To High Cholesterol In Premenopausal Women Aged 40-51 Years Old? J Prev Med Vol.3 No.3: 3. Copyright: © 2018 Homeyer S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited. Introduction Abstract Increased serum cholesterol levels is a major risk factor for coronary heart disease (CHD) [1], and over seventy-one million Purpose: Alcohol use and cholesterol are related in men and American adults have high cholesterol (27%) [2,3]. In American postmenopausal women but relations between alcohol use women, cholesterol levels have been shown to increase with and cholesterol are unclear for premenopausal women. The age, and nearly 1 in 2 American women has high or borderline purpose of this study was to determine whether alcohol use was related to cholesterol in women aged 40-51 years old. -

Herbal Insomnia Medications That Target Gabaergic Systems: a Review of the Psychopharmacological Evidence
Send Orders for Reprints to [email protected] Current Neuropharmacology, 2014, 12, 000-000 1 Herbal Insomnia Medications that Target GABAergic Systems: A Review of the Psychopharmacological Evidence Yuan Shia, Jing-Wen Donga, Jiang-He Zhaob, Li-Na Tanga and Jian-Jun Zhanga,* aState Key Laboratory of Bioactive Substance and Function of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, P.R. China; bDepartment of Pharmacology, School of Marine, Shandong University, Weihai, P.R. China Abstract: Insomnia is a common sleep disorder which is prevalent in women and the elderly. Current insomnia drugs mainly target the -aminobutyric acid (GABA) receptor, melatonin receptor, histamine receptor, orexin, and serotonin receptor. GABAA receptor modulators are ordinarily used to manage insomnia, but they are known to affect sleep maintenance, including residual effects, tolerance, and dependence. In an effort to discover new drugs that relieve insomnia symptoms while avoiding side effects, numerous studies focusing on the neurotransmitter GABA and herbal medicines have been conducted. Traditional herbal medicines, such as Piper methysticum and the seed of Zizyphus jujuba Mill var. spinosa, have been widely reported to improve sleep and other mental disorders. These herbal medicines have been applied for many years in folk medicine, and extracts of these medicines have been used to study their pharmacological actions and mechanisms. Although effective and relatively safe, natural plant products have some side effects, such as hepatotoxicity and skin reactions effects of Piper methysticum. In addition, there are insufficient evidences to certify the safety of most traditional herbal medicine. In this review, we provide an overview of the current state of knowledge regarding a variety of natural plant products that are commonly used to treat insomnia to facilitate future studies. -

E the Effect of Topiramate on Weight Loss in Patients with Type 2 Diabetes
E The effect of topiramate on weight loss in patients with type 2 diabetes RTICL Sedighe Moradi, Scott Reza Jafarian Kerman, Mina Mollabashi A Institute of Endocrinology and Metabolism (Hemmat Campus), Firoozgar Hospital, Tehran University of Medical Sciences, Tehran, Iran Background: Obesity has been associated with several co‑morbidities such as diabetes and increased mortality. In general, the use of medication promotes only a modest weight loss in the range of 2 to 10 kg, usually most effective during the first 6 months of therapy; however, studies have shown positive effects on other risk factors such as blood pressure and serum glucose levels, but there are fewer studies in patients with diabetes. The aim of this study was to assess the effect of topiramate on weight reduction patients with type 2 diabetes. Materials and Methods: This was a 32‑week randomized clinical trial study of 69 subjects during 2008‑2010. RIGINAL Patients, in two treatment groups were given topiramate (39 patients) and Placebo (30 patients) and were subjected to participation in a non‑pharmacologic lifestyle intervention program; which were randomly allocated in our two groups. The percentage change in body O weight and Body Mass Index (BMI) at the end of the study was the primary efficacy endpoint and secondary indicators were changes in blood pressure (BP), proportion of subjects who achieved 5% or 10% weight loss, changes in lipid profile (total cholesterol, low‑density lipoprotein cholesterol, high‑density lipoprotein cholesterol, triglycerides); and changes in glycosylated hemoglobin (HgA1c). Paired samples and independent samples t‑test was used for statistical analysis. -

Central Valley Toxicology Drug List
Chloroform ~F~ Lithium ~A~ Chlorpheniramine Loratadine Famotidine Acebutolol Chlorpromazine Lorazepam Fenoprofen Acetaminophen Cimetidine Loxapine Fentanyl Acetone Citalopram LSD (Lysergide) Fexofenadine 6-mono- Clomipramine acetylmorphine Flecainide ~M~ Clonazepam a-Hydroxyalprazolam Fluconazole Maprotiline Clonidine a-Hydroxytriazolam Flunitrazepam MDA Clorazepate Albuterol Fluoxetine MDMA Clozapine Alprazolam Fluphenazine Medazepam Cocaethylene Amantadine Flurazepam Meperidine Cocaine 7-Aminoflunitrazepam Fluvoxamine Mephobarbital Codeine Amiodarone Fosinopril Meprobamate Conine Amitriptyline Furosemide Mesoridazine Cotinine Amlodipine Methadone Cyanide ~G~ Amobarbital Methanol Cyclobenzaprine Gabapentin Amoxapine d-Methamphetamine Cyclosporine GHB d-Amphetamine l-Methamphetamine Glutethamide l-Amphetamine ~D~ Methapyrilene Guaifenesin Aprobarbital Demoxepam Methaqualone Atenolol Desalkylfurazepam ~H~ Methocarbamol Atropine Desipramine Halazepam Methylphenidate ~B~ Desmethyldoxepin Haloperidol Methyprylon Dextromethoraphan Heroin Metoclopramide Baclofen Diazepam Hexobarbital Metoprolol Barbital Digoxin Hydrocodone Mexiletine Benzoylecgonine Dihydrocodein Hydromorphone Midazolam Benzphetamine Dihydrokevain Hydroxychloroquine Mirtazapine Benztropine Diltiazem Hydroxyzine Morphine (Total/Free) Brodificoum Dimenhydrinate Bromazepam ~N~ Diphenhydramine ~I~ Bupivacaine Nafcillin Disopyramide Ibuprofen Buprenorphine Naloxone Doxapram Imipramine Bupropion Naltrexone Doxazosin Indomethacin Buspirone NAPA Doxepin Isoniazid Butabarbital Naproxen