Ccjm.88A.Ccc076.Full.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Virality Project Weekly Briefing #18 April 20, 2021 - April 27, 2021
Virality Project Weekly Briefing #18 April 20, 2021 - April 27, 2021 This report was created by analysts from the Virality Project, a coalition of research entities focused on real-time detection, analysis, and response to COVID-19 anti-vaccine mis- and disinformation. The Virality Project aims to support information exchange between public health officials, government, and social media platforms through weekly briefings and real-time incident response. Public officials and health organizations interested in officially joining this collaboration can reach the partnership at [email protected]. In this briefing: Events This Week ● Vaccine “shedding” narrative about menstrual cycles creates confusion about COVID-19 vaccines’ impact on women’s health among anti-vaccine and wellness communities. ● Israeli study with small sample size finds correlation between Pfizer vaccine and shingles. Online users twist the findings to suggest a link between vaccines and herpes. ● CDC reports 5,800 “breakthrough” cases of people getting coronavirus after vaccination. Some accounts have seized on this data to suggest that vaccines are ineffective. ● An unfounded claim that a 12-year-old was hospitalized due to a vaccine trial spread among anti-vaccine groups resulting in safety concerns about vaccinating children. ● Anti-vaccine accounts attempt to hijack #RollUpYourSleeves after NBC TV Special to draw attention to vaccine misinformation. Non-English Language ● Chinese language video from Guo Wengui spreads false narrative and Foreign Spotlight that no COVID-19 vaccines work. ● Children’s Health Defense article translated into Chinese spreads on WeChat. ● Report from Israeli People’s Committee uses unverified reports to claim Pfizer vaccine more dangerous than others Ongoing Themes and ● New film featuring footage from the “Worldwide Rally for Tactics Freedom” marketed widely online among anti-vaccine and health freedom groups. -

Testimony of Nadera Lopez-Garrity Oppose S.C.R. No. 10 Health, Human Services and Medicaid Committee February 26, 2020
Testimony of Nadera Lopez-Garrity Oppose S.C.R. No. 10 Health, Human Services and Medicaid Committee February 26, 2020 Chairman Burke, Vice Chair Huffman, Ranking Minority Member Antonio and honorable members of the Health, Human Services and Medicaid Committee. Thank you for the opportunity to speak today in opposition of Senate Concurrent Resolution 10. My name is Nadera Lopez-Garrity and I am the mother of a child whose overall wellbeing compels me to be here today. I oppose S.C.R. No. 10 due to concerning proposed rule changes in the Ohio Administrative Code (OAC) 3701-36-05. I particularly oppose the financial incentives to penalize county health departments whose citizens decline a pharmaceutical product and the intention of publicly posting the rates of consuming such biologics, which not only has no bearing on true immunity but such disclosure would be in violation of Ohioans constitutional rights. The proposed OAC 3701-36-05 rules outlined in paragraph B, section 10, seeks to increase the vaccination rates of children entering kindergarten, seventh and twelfth grades in accordance to section 331.671 of the Revised Code via financial incentives. Financially penalizing county health departments unless they meet such standards will not only lead to coercive tactics that are highly unethical and in violation of the Nuremberg code but fails to address the shortcomings of a practice that is not bullet-proof in neither preventing disease nor promoting good health, both of which the ODH has been entrusted to protect and improve, as they proudly assert in their mission statement. The scientifically sound realities of primary, secondary and tertiary vaccine failure must be taken into consideration if we are to concern ourselves with the prevention of disease, promotion of good health outcomes and the assurance of “safe and healthy environments.” We recently witnessed this reality last fall when there was an outbreak of Bordetella pertussis (whooping cough) in Warren County Ohio. -

An Overview of COVID Vaccine Clinical Trial Results & Some Challenges
An overview of COVID vaccine clinical trial results & some challenges DCVMN Webinar December 8th, 2020 Access to COVID-19 tools ACCESSACCESS TO TOCOVID-19 COVID-19 TOOLS TOOLS (ACT) (ACT) ACCELERATOR ACCELERATOR (ACT) accelerator A GlobalA GlobalCollaboration Collaboration to Accelerate tothe AccelerateDevelopment, the Production Development, and Equitable Production Access to New and Equitable AccessCOVID-19 to New diagnostics, COVID-19 therapeutics diagnostics, and vaccines therapeutics and vaccines VACCINES DIAGNOSTICS THERAPEUTICS (COVAX) Development & Manufacturing Led by CEPI, with industry Procurement and delivery at scale Led by Gavi Policy and allocation Led by WHO Key players SOURCE: (ACT) ACCELERATOR Commitment and Call to Action 24th April 2020 ACT-A / COVAX governance COVAX COORDINATION MEETING CEPI Board Co-Chair: Jane Halton Co-Chair: Dr. Ngozi Gavi Board Workstream leads + DCVMN and IFPMA-selected Reps As needed – R&D&M Chair; COVAX IPG Chair Development & Manufacturing Procurement and delivery Policy and allocation (COVAX) at scale Led by (with industry) Led by Led by R&D&M Investment Committee COVAX Independent Product Group Technical Review Group Portfolio Group Vaccine Teams SWAT teams RAG 3 COVAX SWAT teams are being set up as a joint platform to accelerate COVID- 19 Vaccine development and manufacturing by addressing common challenges together Timely and targeted Multilateral Knowledge-based Resource-efficient Addresses specific cross- Establishes a dialogue Identifies and collates Coordinates between developer technical and global joint effort most relevant materials different organizations/ challenges as they are across different COVID-19 and insights across the initiatives to limit raised and/or identified vaccines organizations broader COVID-19 duplications and ensure on an ongoing basis (incl. -

Dissemination of Vaccine Misinformation on Twitter and Its Countermeasures
Dissertation Dissemination of Vaccine Misinformation on Twitter and Its Countermeasures Christine Chen This document was submitted as a dissertation in March 2021 in partial fulfillment of the requirements of the doctoral degree in public policy analysis at the Pardee RAND Graduate School. The faculty committee that supervised and approved the dissertation consisted of Luke Matthews (Chair), Sarah Nowak and Jeremy Miles. The external reader was Jennifer Golbeck. This dissertation was generously supported by the Anne and James Rothenberg Dissertation Award. PARDEE RAND GRADUATE SCHOOL For more information on this publication, visit http://www.rand.org/pubs/rgs_dissertations/RGSDA1332-1.html Published 2021 by the RAND Corporation, Santa Monica, Calif. is a registered trademarK Limited Print and Electronic Distribution Rights This document and trademarK(s) contained herein are protected by law. This representation of RAND intellectual property is provided for noncommercial use only. Unauthorized posting of this publication online is prohibited. Permission is given to duplicate this document for personal use only, as long as it is unaltered and complete. Permission is reQuired from RAND to reproduce, or reuse in another form, any of its research documents for commercial use. For information on reprint and linking permissions, please visit www.rand.org/pubs/permissions.html. The RAND Corporation is a research organization that develops solutions to public policy challenges to help maKe communities throughout the world safer and more secure, healthier and more prosperous. RAND is nonprofit, nonpartisan, and committed to the public interest. RAND’s publications do not necessarily reflect the opinions of its research clients and sponsors. Support RAND MaKe a tax-deductible charitable contribution at www.rand.org/giving/contribute www.rand.org Abstract Outbreaks of vaccine preventable diseases have continued to affect many parts of the United States. -

SARS-Cov-2 Vaccines in Kidney Transplant Recipients: Will They Be Safe and Effective and How Will We Know?
PERSPECTIVES www.jasn.org SARS-CoV-2 Vaccines in Kidney Transplant Recipients: Will They Be Safe and Effective and How Will We Know? Madeleine R. Heldman and Ajit P. Limaye Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington JASN 32: ccc–ccc, 2021. doi: https://doi.org/10.1681/ASN.2021010023 Coronavirus disease 2019 (COVID-19) will far outweigh risks of vaccination. viral vector–based vaccines could be- has had a major effect on kidney and Accordingly, current guidance from come replication competent and cause other solid organ transplant recipients.1 multiple professional organizations disease, especially in immunocompro- In addition to public health measures, recommend vaccination for all eligible mised individuals. For example, in cells improved access to testing, and thera- organ transplant recipients.2,4,5 concurrently infected with two different peutic developments, vaccination has Each vaccine platform has distinct AdVes, homologous recombination of emerged as a key tool for controlling safety considerations for kidney transplant genetic elements could occur and result the ongoing pandemic. In December recipients. Live (replication-competent) in the emergence of new, pathogenic, 2020, multiple regulatory agencies vaccines are generally contraindicated in replication-competent AdV types.9 This worldwide authorized the use of two immunocompromised individuals be- has been observed in patients with ad- mRNAvaccines for severe acute respiratory cause of a risk of vaccine-acquired -

Immunological Considerations for COVID-19 Vaccine Strategies
REVIEWS Immunological considerations for COVID-19 vaccine strategies Mangalakumari Jeyanathan1,2,3,5, Sam Afkhami1,2,3,5, Fiona Smaill2,3, Matthew S. Miller1,3,4, Brian D. Lichty 1,2 ✉ and Zhou Xing 1,2,3 ✉ Abstract | The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2) is the most formidable challenge to humanity in a century. It is widely believed that prepandemic normalcy will never return until a safe and effective vaccine strategy becomes available and a global vaccination programme is implemented successfully. Here, we discuss the immunological principles that need to be taken into consideration in the development of COVID-19 vaccine strategies. On the basis of these principles, we examine the current COVID-19 vaccine candidates, their strengths and potential shortfalls, and make inferences about their chances of success. Finally, we discuss the scientific and practical challenges that will be faced in the process of developing a successful vaccine and the ways in which COVID-19 vaccine strategies may evolve over the next few years. The coronavirus disease 2019 (COVID-19) outbreak constitute a safe and immunologically effective COVID-19 was first reported in Wuhan, China, in late 2019 and, at vaccine strategy, how to define successful end points the time of writing this article, has since spread to 216 in vaccine efficacy testing and what to expect from countries and territories1. It has brought the world to a the global vaccine effort over the next few years. This standstill. The respiratory viral pathogen severe acute Review outlines the guiding immunological principles respiratory syndrome coronavirus 2 (SARS-CoV-2) has for the design of COVID-19 vaccine strategies and anal- infected at least 20.1 million individuals and killed more yses the current COVID-19 vaccine landscape and the than 737,000 people globally, and counting1. -
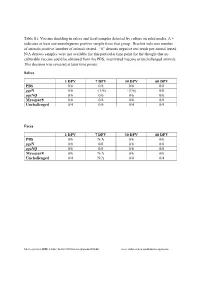
Table S1. Vaccine Shedding in Saliva and Fecal Samples Detected by Culture on Solid Media. a + Indicates at Least One Unambiguous Positive Sample from That Group
Table S1. Vaccine shedding in saliva and fecal samples detected by culture on solid media. A + indicates at least one unambiguous positive sample from that group. Bracket indicates number of animals positive /number of animals tested. “0” denotes negative test result per animal tested. N/A denotes samples were not available for this particular time point for the thought that no culturable vaccine could be obtained from the PBS, inactivated vaccine or unchallenged animals. This decision was reversed at later time points. Saliva 1 DPV 7 DPV 30 DPV 60 DPV PBS 0/6 0/6 0/6 0/6 pgsN 0/6 +(1/6) +(1/6) 0/6 pgsNQ 0/6 0/6 0/6 0/6 Mycopar® 0/6 0/6 0/6 0/6 Unchallenged 0/4 0/6 0/4 0/4 Feces 1 DPV 7 DPV 30 DPV 60 DPV PBS 0/6 N/A 0/6 0/6 pgsN 0/6 0/6 0/6 0/6 pgsNQ 0/6 0/6 0/6 0/6 Mycopar® 0/6 N/A 0/6 0/6 Unchallenged 0/4 N/A 0/4 0/4 Microorganisms 2020, 8, 1427; doi:10.3390/microorganisms8091427 www.mdpi.com/journal/microorganisms Table S2. Vaccine shedding in saliva (top) and feces (bottom) detected by IS900 PCR. A + indicates at least one unambiguous positive sample from that group. Bracket indicates number of animals positive /number of animals tested. “0” denotes negative test result per animal tested. N/A denotes samples were not available for this particular time point for the thought that no culturable vaccine could be obtained from the PBS, inactivated vaccine or naïve animals. -

Viral Shedding from Vaccines Mass Immunizations in Schools and Communities May Actually Endanger the Immune Deficient Via Vaccine Shedding
Viral Shedding from Vaccines Mass immunizations in schools and communities May actually endanger the immune deficient via vaccine shedding. -------------------------------------------------------------------------------------------- Shedding is when the live virus that is injected via vaccine, moves through the human body and comes back out in the feces, droplets from the nose, or saliva from the mouth. Anyone who takes care of the child could potentially contract the disease for some time after that child has received certain live vaccines. This was a huge problem with the oral polio vaccine (OPV), and was one of the reasons why it was taken off the market in the US. The OPV is still used in developing counties. Secondary transmission happens fairly often with some of the live virus vaccines. Influenza, Varicella, and Oral Polio Vaccine (OPV) are the most common. On the other hand it may happen very seldom or not ever with the measles and mumps vaccine viruses. Here are the vaccines that shed or have been known to result in secondary transmission: Measles Vaccine - Although secondary transmission of the vaccine virus has never been documented, measles virus RNA has been detected in the urine of the vaccinees as early as 1 day or as late as 14 days after vaccination. (1) In France, measles virus was isolated in a throat swab of a recently vaccinated child 4 days after fever onset. The virus was then further genetically characterized as a vaccine-type virus. (2) Rubella Vaccine - Excretion of small amounts of live attenuated rubella virus from the nose and throat has occurred in the majority of susceptible individuals 7-28 days after vaccination. -

Secretary's Stationary
Ron DeSantis Mission: Governor To protect, promote & improve the health of all people in Florida through integrated Scott A. Rivkees, MD state, county & community efforts. State Surgeon General Vision: To be the Healthiest State in the Nation FRANKLIN and GULF COVID – 19 UPDATE – 8/27/21 For Franklin County updates straight to your phone: text FranklinCV19 to 888777. For Gulf County updates straight to your phone text GULFCOVID to 888777. 1. The best defense against COVID-19 is the vaccine. COVID-19 vaccines are effective and are a critical tool to bring the pandemic under control. The vaccine helps protect you and the health of the broader community. If you still get COVID-19, the vaccine has been proven to reduce the severity of illness, hospitalization, and death. Vaccines are still available through medical providers, the health department and local pharmacies at no cost. 2. People should talk to their medical provider about COVID-19 treatments appropriate for them. About Monoclonal Antibody Therapy. If you are more likely to get very sick from COVID-19, your healthcare provider might recommend that you receive monoclonal antibody therapy. To find a location, go to https://floridahealthcovid19.gov/monoclonal-antibody-therapy/ Monoclonal antibodies are a treatment authorized by the U.S. Food and Drug Administration (FDA) under an Emergency Use Authorization (EUA) for use in adult and pediatric patients (12 and older) who have either been diagnosed or exposed to someone with COVID-19 and are at high risk for progression to severe illness, hospitalization, or death from COVID-19. In clinical trials, this treatment resulted in a 70% reduction in risk for hospitalization and death, and resulted in an 82% reduction in risk for contracting COVID-19 for people who were exposed to the virus by other members of their household. -

Covid-19 Vaccination: the Pros and Cons
Covid-19 vaccination: The pros and cons Odangowei Inetiminebi Ogidi 1, *, Wonyinbarakemi Ladi Berefagha 2 and Ebifanimi Okara 3 1 Department of Biochemistry, Federal Polytechnic Ekowe, Bayelsa State, Nigeria. 2 Department of Maternal and Child Health Nursing, Niger Delta University, Wilberforce Island, Bayelsa State, Nigeria. 3 Research Laboratory, Federal Medical Center, Yenagoa, Bayelsa State, Nigeria. World Journal of Biology Pharmacy and Health Sciences, 2021, 07(01), 015–022 Publication history: Received on 02 June 2021; revised on 05 July 2021; accepted on 08 July 2021 Article DOI: https://doi.org/10.30574/wjbphs.2021.7.1.0072 Abstract The advent of Severe Acute Respiratory Syndrome Corona Virus 2 (SARS-CoV-2) also known as COVID-19 disease and the dynamics of its rapid spread around the globe are unprecedented. Different preventive efforts have been undertaken in response to this global health challenge, amongst them, vaccine development, distribution and dispensation is at the forefront. Vaccines stimulate the body’s immune system against infectious pathogens; hence, they are one of the greatest medical accomplishments and a cornerstone of public health. There is a strong consensus globally that COVID-19 vaccine is likely the most effective approach to sustainably controlling the COVID-19 pandemic. An unprecedented research effort and global coordination has resulted in a rapid development of COVID-19 vaccines. Here, we review the various types, Pros (status of different COVID-19 vaccines, their utility in COVID-19 control and as a tool of herd immunity or protection) and Cons (various side effects, leaky vaccination and vaccine hesitancy) of COVID-19 vaccines. -

Developing Vaccine Access Programs
WORSHIP ACTION COALITION DEVELOPING VACCINE MESSAGING PROGRAMS WORSHIP ACTION INTRODUCTION COALITION Houses of worship and faith-based organizations have often been a cornerstone in the fight against inequities as well as a trusted source of information and guidance during troubled times. The DEVELOPING current health crisis is no exception. Vaccine hesitancy has been a persistent issue in the vaccine roll out process. Houses of worship can play a key role in informing the message development and VACCINE strategy process, as well as delivering those messages to their MESSAGING congregations and extended communities. This playbook is intended to support faith groups in their effort to deliver these key messages about vaccination. It is supplemented PROGRAMS by additional guides on setting up your house of worship as a vaccine site and developing vaccine access programs. These materials are an initiative of the GHC3’s Worship Action Coalition as part of our commitment to help all faiths return safely to their places of worship and to collaborate with faith leaders to find real solutions to the inequities exacerbated by the pandemic. The initial aim of the Worship Action Coalition was to create best practices for worshiping in-person safely. We are now focused on 1) the role of clergy as trusted sources of information on COVID vaccines, and 2) congregations at disproportionate risk for COVID-19 as locations where members and the surrounding community can get vaccinated, and 3) how both clergy and houses of worship can participate effectively in addressing health disparities. Together, we’re learning about the challenges and working to find real solutions. -

An Appraisal of the Current Scenario in Vaccine Research for COVID-19
viruses Review An Appraisal of the Current Scenario in Vaccine Research for COVID-19 Wai Chin Chong 1,†, Dinesh K. Chellappan 2 , Shakti D. Shukla 3, Gregory M. Peterson 4 , Rahul P. Patel 4, Niraj Kumar Jha 5 , Rajaraman D. Eri 6 , Kamal Dua 3 , Murtaza M. Tambuwala 7,* and Madhur D. Shastri 4,6,*,† 1 Department of Molecular and Translational Science, Monash University, Clayton 3168, Australia; [email protected] 2 Department of Life Sciences, School of Pharmacy, International Medical University (IMU), Kuala Lumpur 57000, Malaysia; [email protected] 3 Discipline of Pharmacy, Graduate School of Health, University of Technology Sydney, Sydney 2007, Australia; [email protected] (S.D.S.); [email protected] (K.D.) 4 School of Pharmacy and Pharmacology, University of Tasmania, Hobart 7005, Australia; [email protected] (G.M.P.); [email protected] (R.P.P.) 5 Department of Biotechnology, School of Engineering & Technology (SET), Sharda University, Greater Noida 201310, UP, India; [email protected] 6 School of Health Sciences, College of Health and Medicine, University of Tasmania, Launceston 7248, Australia; [email protected] 7 School of Pharmacy and Pharmaceutical Science, Ulster University, Coleraine BT52 1SA, UK * Correspondence: [email protected] (M.T.); [email protected] (M.D.S.) † These authors contributed equally to this work. Citation: Chong, W.C.; Chellappan, D.K.; Shukla, S.D.; Peterson, G.M.; Abstract: The recent coronavirus disease 2019 (COVID-19) outbreak has drawn global attention, Patel, R.P.; Jha, N.K.; Eri, R.D.; Dua, affecting millions, disrupting economies and healthcare modalities.