MMWR, Volume 70, Issue 20 — May 21, 2021
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2021-2022 Custom & Standard Information Due Dates
2021-2022 CUSTOM & STANDARD INFORMATION DUE DATES Desired Cover All Desired Cover All Delivery Date Info. Due Text Due Delivery Date Info. Due Text Due May 31 No Deliveries No Deliveries July 19 April 12 May 10 June 1 February 23 March 23 July 20 April 13 May 11 June 2 February 24 March 24 July 21 April 14 May 12 June 3 February 25 March 25 July 22 April 15 May 13 June 4 February 26 March 26 July 23 April 16 May 14 June 7 March 1 March 29 July 26 April 19 May 17 June 8 March 2 March 30 July 27 April 20 May 18 June 9 March 3 March 31 July 28 April 21 May 19 June 10 March 4 April 1 July 29 April 22 May 20 June 11 March 5 April 2 July 30 April 23 May 21 June 14 March 8 April 5 August 2 April 26 May 24 June 15 March 9 April 6 August 3 April 27 May 25 June 16 March 10 April 7 August 4 April 28 May 26 June 17 March 11 April 8 August 5 April 29 May 27 June 18 March 12 April 9 August 6 April 30 May 28 June 21 March 15 April 12 August 9 May 3 May 28 June 22 March 16 April 13 August 10 May 4 June 1 June 23 March 17 April 14 August 11 May 5 June 2 June 24 March 18 April 15 August 12 May 6 June 3 June 25 March 19 April 16 August 13 May 7 June 4 June 28 March 22 April 19 August 16 May 10 June 7 June 29 March 23 April 20 August 17 May 11 June 8 June 30 March 24 April 21 August 18 May 12 June 9 July 1 March 25 April 22 August 19 May 13 June 10 July 2 March 26 April 23 August 20 May 14 June 11 July 5 March 29 April 26 August 23 May 17 June 14 July 6 March 30 April 27 August 24 May 18 June 15 July 7 March 31 April 28 August 25 May 19 June 16 July 8 April 1 April 29 August 26 May 20 June 17 July 9 April 2 April 30 August 27 May 21 June 18 July 12 April 5 May 3 August 30 May 24 June 21 July 13 April 6 May 4 August 31 May 25 June 22 July 14 April 7 May 5 September 1 May 26 June 23 July 15 April 8 May 6 September 2 May 27 June 24 July 16 April 9 May 7 September 3 May 28 June 25. -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -
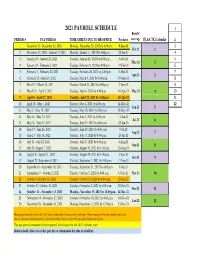
Payroll Calendar 2021
2021 PAYROLL SCHEDULE 1 Benefit PERIOD # PAY PERIOD TIME SHEETS DUE TO HR OFFICE Paydates coverage FLSA 7K Calendar 2 1 December 13- December 26, 2020 Monday, December 28, 2020 by 4:00 p.m. 8-Jan-21 3 Feb-21 1 2 December 27, 2020 - Janurary 9, 2021 Monday, January 11, 2021 by 4:00 p.m. 22-Jan-21 4 3 January 10 - January 23, 2021 Tuesday, January 26, 2021 by 4:00 p.m. 5-Feb-21 5 Mar-21 2 4 January 24 - February 6, 2021 Tuesday, February 9, 2021 by 4:00 p.m. 19-Feb-21 6 5 February 7 - February 20, 2021 Tuesday, February 26, 2021 by 4:00 p.m. 5-Mar-21 7 Apr-21 3 6 February 21 - March 6, 2021 Tuesday, March 9, 2021 by 4:00 p.m. 19-Mar-21 8 7 March 7 - March 20, 2021 Tuesday, March 23, 2021 by 4:00 p.m. 2-Apr-21 9 8 March 21 - April 3, 2021 Tuesday, April 6, 2021 by 4:00 p.m. 16-Apr-21 May-21 4 10 9 April 4 - April 17, 2021 Tuesday, April 20, 2021 by 4:00 p.m. 30-Apr-21 11 10 April 18 - May 1, 2021 Tuesday, May 4, 2021 by 4:00 p.m. 14-May-21 12 Jun-21 5 11 May 2 - May 15, 2021 Tuesday, May 18, 2021 by 4:00 p.m. 28-May-21 12 May 16 - May 29, 2021 Tuesday, June 1, 2021 by 4:00 p.m. 11-Jun-21 Jul-21 6 13 May 30 - June 12, 2021 Tuesday, June 15, 2021 by 4:00 p.m. -

May 21 SBE WS Minutes
NEVADA DEPARTMENT OF EDUCATION STATE BOARD OF EDUCATION MEETING WORK SESSION MAY 21, 2021 3:00 PM Office Address City Meeting Room Department of Education 2080 E. Flamingo Rd. Las Vegas Room 114 Department of Education 700 E. Fifth St. Carson City Board Room Department of Education Virtual/Livestream n/a n/a SUMMARY MINUTES OF THE BOARD MEETING BOARD MEMBERS PRESENT In Las Vegas Felicia Ortiz, President Mark Newburn, Vice President Dr. René Cantú Dr. Katie Dockweiler Tamara Hudson Tim Hughes Via Videoconference Katie Coombs Alex Gallegos Cathy McAdoo DEPARTMENT STAFF PRESENT In Carson City Jhone M. Ebert, Superintendent of Public Instruction FACILITATOR In Las Vegas Dr. Debb Oliver, Executive Director, Nevada Association of School Boards Via Videoconference Dr. Summer Stephens, Superintendent, Churchill County School District LEGAL STAFF PRESENT Via Videoconference Greg Ott, Chief Deputy Attorney General AUDIENCE IN ATTENDANCE Via Livestream Page 1 of 7 1: CALL TO ORDER, ROLL CALL, AND PLEDGE OF ALLEGIANCE Meeting called to order at 3:10 P.M. by President Felicia Ortiz. Quorum was established. President Ortiz led the Pledge of Allegiance and provided a land acknowledgement. 2: PUBLIC COMMENT #1 Yvette Williams, Clark County Black Caucus, submitted public comment regarding State Board Goals and Recommendations. (A complete copy of the statement is available in Appendix A) Educate Nevada Now submitted public comment regarding State Board Goals and Recommendations. (A complete copy of the statement is available in Appendix A) Erik Harsh and Ysa Cruz, students of Douglas County School District, submitted public comment regarding LGBTQ+ curriculum. (A complete copy of the statement is available in Appendix A) 3: INFORMATION, DISCUSSION, AND POSSIBLE ACTION REGARDING SETTING STATE BOARD OF EDUCATION GOALS(S) IN ACCORDANCE WITH SILVER STATE GOVERNANCE TRAINING Felicia Ortiz, President, State Board of Education; Jhone M. -

Flex Dates.Xlsx
1st Day 1st Day of Your Desired Stay you may Call January 3, 2021 ↔ November 4, 2020 January 4, 2021 ↔ November 5, 2020 January 5, 2021 ↔ November 6, 2020 January 6, 2021 ↔ November 7, 2020 January 7, 2021 ↔ November 8, 2020 January 8, 2021 ↔ November 9, 2020 January 9, 2021 ↔ November 10, 2020 January 10, 2021 ↔ November 11, 2020 January 11, 2021 ↔ November 12, 2020 January 12, 2021 ↔ November 13, 2020 January 13, 2021 ↔ November 14, 2020 January 14, 2021 ↔ November 15, 2020 January 15, 2021 ↔ November 16, 2020 January 16, 2021 ↔ November 17, 2020 January 17, 2021 ↔ November 18, 2020 January 18, 2021 ↔ November 19, 2020 January 19, 2021 ↔ November 20, 2020 January 20, 2021 ↔ November 21, 2020 January 21, 2021 ↔ November 22, 2020 January 22, 2021 ↔ November 23, 2020 January 23, 2021 ↔ November 24, 2020 January 24, 2021 ↔ November 25, 2020 January 25, 2021 ↔ November 26, 2020 January 26, 2021 ↔ November 27, 2020 January 27, 2021 ↔ November 28, 2020 January 28, 2021 ↔ November 29, 2020 January 29, 2021 ↔ November 30, 2020 January 30, 2021 ↔ December 1, 2020 January 31, 2021 ↔ December 2, 2020 February 1, 2021 ↔ December 3, 2020 February 2, 2021 ↔ December 4, 2020 1st Day 1st Day of Your Desired Stay you may Call February 3, 2021 ↔ December 5, 2020 February 4, 2021 ↔ December 6, 2020 February 5, 2021 ↔ December 7, 2020 February 6, 2021 ↔ December 8, 2020 February 7, 2021 ↔ December 9, 2020 February 8, 2021 ↔ December 10, 2020 February 9, 2021 ↔ December 11, 2020 February 10, 2021 ↔ December 12, 2020 February 11, 2021 ↔ December 13, 2020 -

Monday, May 10, 2021 WHERE WE ARE TODAY 2
Monday, May 10, 2021 WHERE WE ARE TODAY 2 May 10, 2021 We have made free COVID-19 testing 3 broadly accessible. COVID-19 tests are free, walk-up sites are open across DC, and now at- home testing kits are available. May 10, 2021 We established and ramped up a 4 Contact Trace Force. Contact tracers reach out to 98.1% of positive cases within 1 day, and 99.8% of close contacts within 2 days. Additionally, nearly 800,000 phones have opt-ed in to DC’s exposure notification tool. May 10, 2021 We mitigated any strain on our 5 hospital system. With the goal of never needing to use it, we built an Alternate Care Site and now we are dismantling it. May 10, 2021 6 SINCE DECEMBER 2020, DC HAS FULLY VACCINATED 226,566 RESIDENTS Vaccines are free and available on-demand at walk-up sites across DC. May 10, 2021 WALK-UP VACCINATION SITES - MAY 2021 7 If you received your first dose in DC, please return to the same location for your second dose. If you received a first dose out of state, you may visit a walk-up location with corresponding vaccine type with your vaccine card and receive your second dose. May 10, 2021 DAILY CASE RATE 8 District of Columbia COVID-19 Daily Case Rate per 100,000 population (7-day average) PHASE 1 PHASE 2 40 n o i t a l u p o p 30 0 May 05 0 0 , 27.56 0 0 1 r e p e 20 t a r e Substantial Community Spread (>15) s a Jul 23 Aug 13 c y l 10.86 10.76 i a May 31 D 10 Moderate Community Spread (5-15) 12.85 Aug 04 9.03Aug 20 May 08 Minimal Community Spread (<5) Jul 05 7.14 6.61 0 4.54 Mar 23, 20 Apr 27, 20 Jun 1, 20 Jul 6, 20 Aug 10, 20 Sep 14, 20 Oct 19, 20 Nov 23, 20 Dec 28, 20 Feb 1, 21 Mar 8, 21 Apr 12, 21 May 17, 21 Date of Public Report Data Source: DC Health. -

Pay Date Calendar
Pay Date Information Select the pay period start date that coincides with your first day of employment. Pay Period Pay Period Begins (Sunday) Pay Period Ends (Saturday) Official Pay Date (Thursday)* 1 January 10, 2016 January 23, 2016 February 4, 2016 2 January 24, 2016 February 6, 2016 February 18, 2016 3 February 7, 2016 February 20, 2016 March 3, 2016 4 February 21, 2016 March 5, 2016 March 17, 2016 5 March 6, 2016 March 19, 2016 March 31, 2016 6 March 20, 2016 April 2, 2016 April 14, 2016 7 April 3, 2016 April 16, 2016 April 28, 2016 8 April 17, 2016 April 30, 2016 May 12, 2016 9 May 1, 2016 May 14, 2016 May 26, 2016 10 May 15, 2016 May 28, 2016 June 9, 2016 11 May 29, 2016 June 11, 2016 June 23, 2016 12 June 12, 2016 June 25, 2016 July 7, 2016 13 June 26, 2016 July 9, 2016 July 21, 2016 14 July 10, 2016 July 23, 2016 August 4, 2016 15 July 24, 2016 August 6, 2016 August 18, 2016 16 August 7, 2016 August 20, 2016 September 1, 2016 17 August 21, 2016 September 3, 2016 September 15, 2016 18 September 4, 2016 September 17, 2016 September 29, 2016 19 September 18, 2016 October 1, 2016 October 13, 2016 20 October 2, 2016 October 15, 2016 October 27, 2016 21 October 16, 2016 October 29, 2016 November 10, 2016 22 October 30, 2016 November 12, 2016 November 24, 2016 23 November 13, 2016 November 26, 2016 December 8, 2016 24 November 27, 2016 December 10, 2016 December 22, 2016 25 December 11, 2016 December 24, 2016 January 5, 2017 26 December 25, 2016 January 7, 2017 January 19, 2017 1 January 8, 2017 January 21, 2017 February 2, 2017 2 January -

2021 Park Hours
Holiday World & Splashin' Safari 2021 Operating Calendar May, 2021 Holiday World Hours Splashin' Safari Hours Special Events Saturday, May 1 Holiday World & Splashin' Safari are closed Sunday, May 2 Holiday World & Splashin' Safari are closed Monday, May 3 Holiday World & Splashin' Safari are closed Tuesday, May 4 Holiday World & Splashin' Safari are closed Wednesday, May 5 Holiday World & Splashin' Safari are closed Thursday, May 6 Holiday World & Splashin' Safari are closed Friday, May 7 Holiday World & Splashin' Safari are closed Saturday, May 8 Platinum Season Pass Preview Day: Holiday World is open from 10 AM until 6 PM Splashin' Safari is Closed Platinum Season Passholder Party Sunday, May 9 Passholder Preview Day: Holiday World is open from 10 AM until 6 PM Splashin' Safari is Closed Monday, May 10 Holiday World & Splashin' Safari are closed Tuesday, May 11 Holiday World & Splashin' Safari are closed Wednesday, May 12 Holiday World & Splashin' Safari are closed Thursday, May 13 Holiday World & Splashin' Safari are closed Friday, May 14 Holiday World & Splashin' Safari are closed for a private outing Saturday, May 15 Holiday World is open from 10 AM until 4 PM Splashin' Safari is Closed Sunday, May 16 Holiday World is open from 10 AM until 4 PM Splashin' Safari is Closed Monday, May 17 Holiday World & Splashin' Safari are closed Tuesday, May 18 Holiday World & Splashin' Safari are closed Wednesday, May 19 Holiday World & Splashin' Safari are closed Thursday, May 20 Holiday World & Splashin' Safari are closed Friday, May 21 Holiday -

Resolution No.: 2021-______
RESOLUTION NO.: 2021-______ WHEREAS, the Mayor and Council of the City of Salem met from time to time in Executive Session for purposes authorized by the Illinois Open Meetings Act; and WHEREAS, as required by the Act, the City Clerk has kept written minutes of all such executive sessions; and WHEREAS, pursuant to the requirements of Public Act 85-1355, the Mayor and Council have met in closed session to review all closed session minutes; and WHEREAS, they have determined that a need for confidentiality still exists as to the executive session minutes from the meetings set forth on Schedule "A" attached hereto; and WHEREAS, they have further determined that the minutes of the meetings listed on Schedule "B" attached hereto no longer require confidential treatment and should be made available for public inspection; NOW, THEREFORE, BE IT RESOLVED BY THE MAYOR AND COUNCIL OF THE CITY OF SALEM, MARION COUNTY, ILLINOIS, as follows: SECTION 1: The Executive Session Minutes from those meetings set forth on Schedule "B" attached hereto are hereby released. SECTION 2: The City Clerk is hereby authorized and directed to make said minutes available for inspection and copying in accordance with the standing procedures of the Clerk's Office. 1 SECTION 3: This Resolution shall be in full force and effect from and after its passage. IN WITNESS WHEREOF, I have hereunto set my hand and caused the seal of the City of Salem, Illinois, to be affixed this 6th day of July, 2021. CITY OF SALEM, ILLINOIS MAYOR ATTEST: CITY CLERK 2 SCHEDULE A EXECUTIVE SESSION -

114-Day Swine Gestation Table
114-DAY SWINE GESTATION TABLE BREEDING DUE BREEDING DUE BREEDING DUE BREEDING DUE BREEDING DUE BREEDING DUE DATE DATE DATE DATE DATE DATE DATE DATE DATE DATE DATE DATE Jan 1 Apr 25 Mar 1 Jun 23 May 1 Aug 23 Jul 1 Oct 23 Sep 1 Dec 24 Nov 1 Feb 23 Jan 2 Apr 26 Mar 2 Jun 24 May 2 Aug 24 Jul 2 Oct 24 Sep 2 Dec 25 Nov 2 Feb 24 Jan 3 Apr 27 Mar 3 Jun 25 May 3 Aug 25 Jul 3 Oct 25 Sep 3 Dec 26 Nov 3 Feb 25 Jan 4 Apr 28 Mar 4 Jun 26 May 4 Aug 26 Jul 4 Oct 26 Sep 4 Dec 27 Nov 4 Feb 26 Jan 5 Apr 29 Mar 5 Jun 27 May 5 Aug 27 Jul 5 Oct 27 Sep 5 Dec 28 Nov 5 Feb 27 Jan 6 Apr 30 Mar 6 Jun 28 May 6 Aug 28 Jul 6 Oct 28 Sep 6 Dec 29 Nov 6 Feb 28 Jan 7 May 1 Mar 7 Jun 29 May 7 Aug 29 Jul 7 Oct 29 Sep 7 Dec 30 Nov 7 Mar 1 Jan 8 May 2 Mar 8 Jun 30 May 8 Aug 30 Jul 8 Oct 30 Sep 8 Dec 31 Nov 8 Mar 2 Jan 9 May 3 Mar 9 Jul 1 May 9 Aug 31 Jul 9 Oct 31 Sep 9 Jan 1 Nov 9 Mar 3 Jan 10 May 4 Mar 10 Jul 2 May 10 Sep 1 Jul 10 Nov 1 Sep 10 Jan 2 Nov 10 Mar 4 Jan 11 May 5 Mar 11 Jul 3 May 11 Sep 2 Jul 11 Nov 2 Sep 11 Jan 3 Nov 11 Mar 5 Jan 12 May 6 Mar 12 Jul 4 May 12 Sep 3 Jul 12 Nov 3 Sep 12 Jan 4 Nov 12 Mar 6 Jan 13 May 7 Mar 13 Jul 5 May 13 Sep 4 Jul 13 Nov 4 Sep 13 Jan 5 Nov 13 Mar 7 Jan 14 May 8 Mar 14 Jul 6 May 14 Sep 5 Jul 14 Nov 5 Sep 14 Jan 6 Nov 14 Mar 8 Jan 15 May 9 Mar 15 Jul 7 May 15 Sep 6 Jul 15 Nov 6 Sep 15 Jan 7 Nov 15 Mar 9 Jan 16 May 10 Mar 16 Jul 8 May 16 Sep 7 Jul 16 Nov 7 Sep 16 Jan 8 Nov 16 Mar 10 Jan 17 May 11 Mar 17 Jul 9 May 17 Sep 8 Jul 17 Nov 8 Sep 17 Jan 9 Nov 17 Mar 11 Jan 18 May 12 Mar 18 Jul 10 May 18 Sep 9 Jul 18 Nov 9 Sep 18 Jan 10 Nov -

Resort-Calendar.Pdf
Week 2020 2021 2022 2023 2024 no 1 10-Jan - 17-Jan 8-Jan - 15-Jan 7-Jan - 14-Jan 6-Jan - 13-Jan 5-Jan - 12-Jan 2 17-Jan - 24-Jan 15-Jan - 22-Jan 14-Jan - 21-Jan 13-Jan - 20-Jan 12-Jan - 19-Jan 3 24-Jan - 31-Jan 22-Jan - 29-Jan 21-Jan - 28-Jan 20-Jan - 27-Jan 19-Jan - 26-Jan 4 31-Jan - 7-Feb 29-Jan - 5-Feb 28-Jan - 4-Feb 27-Jan - 3-Feb 26-Jan - 2-Feb 5 7-Feb - 14-Feb 5-Feb - 12-Feb 4-Feb - 11-Feb 3-Feb - 10-Feb 2-Feb - 9-Feb 6 14-Feb - 21-Feb 12-Feb - 19-Feb 11-Feb - 18-Feb 10-Feb - 17-Feb 9-Feb - 16-Feb 7 21-Feb - 28-Feb 19-Feb - 26-Feb 18-Feb - 25-Feb 17-Feb - 24-Feb 16-Feb - 23-Feb 8 28-Feb - 6-Mar 26-Feb - 5-Mar 25-Feb - 4-Mar 24-Feb - 3-Mar 23-Feb - 1-Mar 9 6-Mar - 13-Mar 5-Mar - 12-Mar 4-Mar - 11-Mar 3-Mar - 10-Mar 1-Mar - 8-Mar 10 13-Mar - 20-Mar 12-Mar - 19-Mar 11-Mar - 18-Mar 10-Mar - 17-Mar 8-Mar - 15-Mar 11 20-Mar - 27-Mar 19-Mar - 26-Mar 18-Mar - 25-Mar 17-Mar - 24-Mar 15-Mar - 22-Mar 12 27-Mar - 3-Apr 26-Mar - 2-Apr 25-Mar - 1-Apr 24-Mar - 31-Mar 22-Mar - 29-Mar 13 3-Apr - 10-Apr 2-Apr - 9-Apr 1-Apr - 8-Apr 31-Mar - 7-Apr 29-Mar - 5-Apr 14 10-Apr - 17-Apr 9-Apr - 16-Apr 8-Apr - 15-Apr 7-Apr - 14-Apr 5-Apr - 12-Apr 15 17-Apr - 24-Apr 16-Apr - 23-Apr 15-Apr - 22-Apr 14-Apr - 21-Apr 12-Apr - 19-Apr 16 24-Apr - 1-May 23-Apr - 30-Apr 22-Apr - 29-Apr 21-Apr - 28-Apr 19-Apr - 26-Apr 17 1-May - 8-May 30-Apr - 7-May 29-Apr - 6-May 28-Apr - 5-May 26-Apr - 3-May 18 8-May - 15-May 7-May - 14-May 6-May - 13-May 5-May - 12-May 3-May - 10-May 19 15-May - 22-May 14-May - 21-May 13-May - 20-May 12-May - 19-May 10-May - 17-May 20 22-May - 29-May -

Date of Close Contact Exposure
Date of Close Contact Exposure 7 days 10 days 14 days Monday, November 16, 2020 Tuesday, November 24, 2020 Friday, November 27, 2020 Tuesday, December 1, 2020 Tuesday, November 17, 2020 Wednesday, November 25, 2020 Saturday, November 28, 2020 Wednesday, December 2, 2020 Wednesday, November 18, 2020 Thursday, November 26, 2020 Sunday, November 29, 2020 Thursday, December 3, 2020 Thursday, November 19, 2020 Friday, November 27, 2020 Monday, November 30, 2020 Friday, December 4, 2020 Friday, November 20, 2020 Saturday, November 28, 2020 Tuesday, December 1, 2020 Saturday, December 5, 2020 Saturday, November 21, 2020 Sunday, November 29, 2020 Wednesday, December 2, 2020 Sunday, December 6, 2020 Sunday, November 22, 2020 Monday, November 30, 2020 Thursday, December 3, 2020 Monday, December 7, 2020 Monday, November 23, 2020 Tuesday, December 1, 2020 Friday, December 4, 2020 Tuesday, December 8, 2020 Tuesday, November 24, 2020 Wednesday, December 2, 2020 Saturday, December 5, 2020 Wednesday, December 9, 2020 Wednesday, November 25, 2020 Thursday, December 3, 2020 Sunday, December 6, 2020 Thursday, December 10, 2020 Thursday, November 26, 2020 Friday, December 4, 2020 Monday, December 7, 2020 Friday, December 11, 2020 Friday, November 27, 2020 Saturday, December 5, 2020 Tuesday, December 8, 2020 Saturday, December 12, 2020 Saturday, November 28, 2020 Sunday, December 6, 2020 Wednesday, December 9, 2020 Sunday, December 13, 2020 Sunday, November 29, 2020 Monday, December 7, 2020 Thursday, December 10, 2020 Monday, December 14, 2020 Monday, November