The Neocortex of Cetartiodactyls: I. a Comparative
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nervous Tissue
Nervous Tissue Prof.Prof. ZhouZhou LiLi Dept.Dept. ofof HistologyHistology andand EmbryologyEmbryology Organization:Organization: neuronsneurons (nerve(nerve cells)cells) neuroglialneuroglial cellscells Function:Function: Ⅰ Neurons 1.1. structurestructure ofof neuronneuron somasoma neuriteneurite a.a. dendritedendrite b.b. axonaxon 1.11.1 somasoma (1)(1) nucleusnucleus LocatedLocated inin thethe centercenter ofof soma,soma, largelarge andand palepale--stainingstaining nucleusnucleus ProminentProminent nucleolusnucleolus (2)(2) cytoplasmcytoplasm (perikaryon)(perikaryon) a.a. NisslNissl bodybody b.b. neurofibrilneurofibril NisslNissl’’ss bodiesbodies LM:LM: basophilicbasophilic massmass oror granulesgranules Nissl’s Body (TEM) EMEM:: RERRER,, freefree RbRb FunctionFunction:: producingproducing thethe proteinprotein ofof neuronneuron structurestructure andand enzymeenzyme producingproducing thethe neurotransmitterneurotransmitter NeurofibrilNeurofibril thethe structurestructure LM:LM: EM:EM: NeurofilamentNeurofilament micmicrotubulerotubule FunctionFunction cytoskeleton,cytoskeleton, toto participateparticipate inin substancesubstance transporttransport LipofuscinLipofuscin (3)(3) CellCell membranemembrane excitableexcitable membranemembrane ,, receivingreceiving stimutation,stimutation, fromingfroming andand conductingconducting nervenerve impulesimpules neurite: 1.2 Dendrite dendritic spine spine apparatus Function: 1.3 Axon axon hillock, axon terminal, axolemma Axoplasm: microfilament, microtubules, neurofilament, mitochondria, -

Meeting Schedule
Track Theme B Bones/Muscle/Connective Tissue C Cardiovascular CB Cell Biology DB Developmental Biology/Morphology ED Education & Teaching EV Evolution/Anthropology I Imaging N Neuroscience PD Career and Professional Development RM Regenerative Medicine (Stem Cells, Tissue Regeneration) V Vertebrate Paleontology All sessions are scheduled eastern time (EDT) ON-DEMAND Career Central On-Demand Short Talks Co-sponsored by AAA’s Profesional Development Committee These on demand talks can be seen at anytime. Establishing Yourself as a Science Educator Darren Hoffman (University of Iowa Carver College of Medicine) In this presentation, you’ll learn strategies for launching a career in science teaching. We’ll explore key elements of the CV that will stand out in your job search, ways to acquire teaching experience when opportunities in your department are scarce, and how to develop your personal identity as a teacher. Negotiate like a Pro Carrie Elzie (Eastern Virginia Medical School) Creating a conducive work environment requires successful negotiation at many levels, with different individuals and unique situations. Thus, negotiation skills are important, not only for salaries, but many other aspects of a career including schedules, resources, and opportunities. In this session, you will learn some brief tips of how to negotiate like a professional including what to do and more importantly, what not to do. #SocialMedia: Personal Branding & Professionalism Mikaela Stiver (University of Toronto) Long gone are the days when social media platforms were just for socializing! Whether you use social media regularly in your professional life or are just getting started, this microlearning talk has something for everyone. We will cover the fundamentals of personal branding, explore a few examples on social media, and discuss the importance of professionalism with an emphasis on anatomical sciences. -

Synaptic Mechanisms of Bipolar Cell Terminals
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Vision Research 39 (1999) 2469–2476 Synaptic mechanisms of bipolar cell terminals Gary Matthews * Department of Neurobiology and Beha6ior, State Uni6ersity of New York, Stony Brook, NY 11794-5230, USA Received 8 May 1998; received in revised form 24 August 1998 Abstract Giant synaptic terminals of goldfish bipolar neurons allow direct studies of presynaptic mechanisms underlying neurotransmit- ter release and its modulation. Calcium influx via L-type calcium channels of the terminal triggers synaptic vesicle exocytosis, which can be monitored in isolated terminals by means of the associated changes in membrane capacitance. Information about the kinetics and calcium dependence of synaptic exocytosis has been obtained from capacitance measurements in these ribbon-type synaptic terminals. © 1999 Elsevier Science Ltd. All rights reserved. Keywords: Synaptic mechanisms; Terminals; Bipolar neurons Studies of presynaptic mechanisms in the vertebrate enzymatic digestion of goldfish retina or in single, central nervous system (CNS) are complicated by the isolated terminals. Consistent with their central role in small size of most CNS synaptic terminals. However, neurotransmitter release, voltage-dependent calcium several notable exceptions allow direct electrical mea- channels are located predominantly in the synaptic surements to be made from single terminals, including terminal of the bipolar cell (Tachibana & Okada, 1991; giant -

Notch-Signaling in Retinal Regeneration and Müller Glial Plasticity
Notch-Signaling in Retinal Regeneration and Müller glial Plasticity DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Kanika Ghai, MS Neuroscience Graduate Studies Program The Ohio State University 2009 Dissertation Committee: Dr. Andy J Fischer, Advisor Dr. Heithem El-Hodiri Dr. Susan Cole Dr. Paul Henion Copyright by Kanika Ghai 2009 ABSTRACT Eye diseases such as blindness, age-related macular degeneration (AMD), diabetic retinopathy and glaucoma are highly prevalent in the developed world, especially in a rapidly aging population. These sight-threatening diseases all involve the progressive loss of cells from the retina, the light-sensing neural tissue that lines the back of the eye. Thus, developing strategies to replace dying retinal cells or prolonging neuronal survival is essential to preserving sight. In this regard, cell-based therapies hold great potential as a treatment for retinal diseases. One strategy is to stimulate cells within the retina to produce new neurons. This dissertation elucidates the properties of the primary support cell in the chicken retina, known as the Müller glia, which have recently been shown to possess stem-cell like properties, with the potential to form new neurons in damaged retinas. However, the mechanisms that govern this stem-cell like ability are less well understood. In order to better understand these properties, we analyze the role of one of the key developmental processes, i.e., the Notch-Signaling Pathway in regulating proliferative, neuroprotective and regenerative properties of Müller glia and bestow them with this plasticity. -

Microcircuitry of Bipolar Cells in Cat Retina1
0270.6474/84/0412-2920$02.00/0 The Journal of Neuroscience Copyright 0 Society for Neuroscience Vol. 4, No. 12, pp. 2920-2938 Printed in U.S.A. December 1984 MICROCIRCUITRY OF BIPOLAR CELLS IN CAT RETINA1 BARBARA A. McGUIRE,’ JOHN K. STEVENS,3 AND PETER STERLING Department of Anatomy, School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania 19104 Received October 31, 1983; Revised June 4, 1984; Accepted June 6, 1984 Abstract We have studied 15 bipolar neurons from a small patch (14 x 120 Frn) of adult cat retina located within the area centralis. From electron micrographs of 189 serial ultrathin sections, the axon of each bipolar cell was substantially reconstructed with its synaptic inputs and outputs by means of a computer-controlled reconstruc- tion system. Based on differences in stratification, cytology, and synaptic connections, we identified eight different cell types among the group of 15 neurons: one type of rod bipolar and seven types of cone bipolar neurons. These types correspond to those identified by the Golgi method and by intracellular recording. Those bipolar cell types for which we reconstructed three or four examples were extremely regular in form, size, and cytology, and also in the quantitative details of their synaptic connections. They appeared quite as specific in these respects as invertebrate “identified” neurons. The synaptic patterns observed for each type of bipolar neuron were complex but may be summarized as follows: the rod bipolar axon ended in sublamina b of the inner plexiform layer and provided major input to the AI1 amacrine cell. The axons of three types of cone bipolar cells also terminated in sublamina b and provided contacts to dendrites of on-6 and other ganglion cells. -

South Georgia and Antarctic Peninsula Earth’S Greatest Wildlife Destination October 21 to November 12, 2021
South Georgia and Antarctic Peninsula Earth’s Greatest Wildlife Destination October 21 to November 12, 2021 King Penguins, South Georgia Island © Scott Davis SAFARI OVERVIEW Experience the vibrant spring of South Georgia Island and the early season of the Antarctic Peninsula. Beneath the towering, snow-blanketed mountains of South Georgia Island, observe and photograph special wildlife behaviors seldom seen. This time of year is the only time you can find southern elephant seal bulls fight for territories while females nurse young, distinctly marked gray-headed albatross attending to their cliffside nests, and awkward wandering albatross young attempting first flight. You’ll stand amongst vast colonies of king penguins and watch macaroni penguins launching into the ocean. This time of year, the Antarctic Peninsula is in the beginnings of its spring season when the ice in the Weddell Sea can open up, allowing opportunities for lone emperor penguins to wander on ice floes. At penguin colonies, you’ll find penguins courting, setting up nests, and perhaps laying eggs. Through over twenty-five years of experience in the Antarctic, we offer the most in-depth exploration of one of the densest wildlife spectacles found anywhere in the world, and with only 100 passengers, you’ll have ample opportunities to experience this spectacle during every landing and Zodiac cruise. Cheesemans’ Ecology Safaris Page 1 of 19 Updated: March 2021 HIGHLIGHTS • Spend six full days on South Georgia Island and six full days in the Antarctic Peninsula and South Shetland Islands with maximum shore time and Zodiac cruising. • See five penguin species (possibly 6)! Plus, many species of whales, seals, albatross, and seabirds. -

The Diverse Roles of Ribbon Synapses in Sensory Neurotransmission
Nature Reviews Neuroscience | AOP, published online 3 November 2010; doi:10.1038/nrn2924 REVIEWS The diverse roles of ribbon synapses in sensory neurotransmission Gary Matthews* and Paul Fuchs‡ Abstract | Sensory synapses of the visual and auditory systems must faithfully encode a wide dynamic range of graded signals, and must be capable of sustained transmitter release over long periods of time. Functionally and morphologically, these sensory synapses are unique: their active zones are specialized in several ways for sustained, rapid vesicle exocytosis, but their most striking feature is an organelle called the synaptic ribbon, which is a proteinaceous structure that extends into the cytoplasm at the active zone and tethers a large pool of releasable vesicles. But precisely how does the ribbon function to support tonic release at these synapses? Recent genetic and biophysical advances have begun to open the ‘black box’ of the synaptic ribbon with some surprising findings and promise to resolve its function in vision and hearing. Changes in our external environment are detected by photoreceptors, electroreceptors, and hair cells of sensory receptor cells, which transduce sensory stim- vesti bular organs and the lateral line system. In the uli into an electrical signal that is graded depending visual system, ribbons are also found at the output on the stimulus intensity. In vision, balance and hear- synapses of the second-order retinal b ipolar neurons, ing, synapses of the receptor cells are unusual because which signal by means of graded changes in mem- they function tonically — that is, they transmit graded brane potential, similar to photoreceptors and hair information with high fidelity across a broad range of cells. -
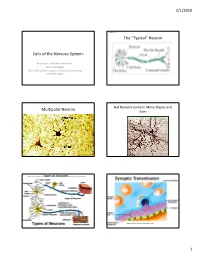
Cells of the Nervous System: the “Typical” Neuron Multipolar Neuron
2/1/2010 Book Fig. 1.1 The “Typical” Neuron Cells of the Nervous System: Neurons: cells that receive & send messages Glia: cells which support neuron functioning in many ways But Neurons Come in Many Shapes and Multipolar Neuron Sizes Types of Neurons Book Fig 1.1 Sensory Neuron Motor Neuron Some proteins serve as receptor sites. 1 2/1/2010 Best Known Neurotransmitters (study handout linked to syllabus) • Acetylcholine (ACh) • Norepinephrine (NE) • Dopamine (DA) • Serotonin or 5-Hydroxytryptamine (5HT) • GABA Released neurotransmitter must bind to specially shaped receptors like a key fitting into a lock. We now know there are multiple subtypes of receptors for each • Glutamate-most widespread excitatory neurotransmitter. transmitter Then the transmitter must be removed from the synapse either by reuptake or enzymatic breakdown. Here’s some background on ACh before we cover an Best Known Neurotransmitters Example of a neurotransmitter related disorder (FYI only – not completely up-to-date list of the number Acetylcholine (ACh) of identified receptor subtypes) • Acetylcholine (ACh) (7 receptor subtypes) • neurons using ACh are known as “cholinergic neurons”. • Examples: Norepinephrine (NE) (11 receptor subtypes) • motor neurons • Dopamine DA) (5 receptor subtypes) • parasympathetic neurons • many CNS neurons (in cortex, basal ganglia, hippocampus, • Serotonin (5HT) (14 receptor subtypes) brainstem) • GABA (2 receptor subtypes) • Different ACh receptor types on muscle (nicotinic) than in the nervous system (muscarinic) • Glutamate (10 receptor -

Anatomical Adaptations of Aquatic Mammals
THE ANATOMICAL RECORD 290:507–513 (2007) Anatomical Adaptations of Aquatic Mammals JOY S. REIDENBERG* Center for Anatomy and Functional Morphology, Department of Medical Education, Mount Sinai School of Medicine, New York, New York ABSTRACT This special issue of the Anatomical Record explores many of the an- atomical adaptations exhibited by aquatic mammals that enable life in the water. Anatomical observations on a range of fossil and living marine and freshwater mammals are presented, including sirenians (manatees and dugongs), cetaceans (both baleen whales and toothed whales, includ- ing dolphins and porpoises), pinnipeds (seals, sea lions, and walruses), the sea otter, and the pygmy hippopotamus. A range of anatomical sys- tems are covered in this issue, including the external form (integument, tail shape), nervous system (eye, ear, brain), musculoskeletal systems (cranium, mandible, hyoid, vertebral column, flipper/forelimb), digestive tract (teeth/tusks/baleen, tongue, stomach), and respiratory tract (larynx). Emphasis is placed on exploring anatomical function in the context of aquatic life. The following topics are addressed: evolution, sound produc- tion, sound reception, feeding, locomotion, buoyancy control, thermoregu- lation, cognition, and behavior. A variety of approaches and techniques are used to examine and characterize these adaptations, ranging from dissection, to histology, to electron microscopy, to two-dimensional (2D) and 3D computerized tomography, to experimental field tests of function. The articles in this issue are a blend of literature review and new, hy- pothesis-driven anatomical research, which highlight the special nature of anatomical form and function in aquatic mammals that enables their exquisite adaptation for life in such a challenging environment. Ó 2007 Wiley-Liss, Inc. -

Terrestrial, Semiaquatic, and Fully Aquatic Mammal Sound Production Mechanisms
Terrestrial, Semiaquatic, and Fully Aquatic Mammal Sound Production Mechanisms Joy S. Reidenberg Aquatic mammals generate sound underwater but use air-driven systems derived from terrestrial ancestors. How do they do it without drowning? Postal: Center for Anatomy and Functional Morphology Terrestrial mammals produce sound in air mainly for communication, while many Icahn School of Medicine at aquatic mammals can communicate by vocalizing in air or underwater. A sub- Mount Sinai set of aquatic mammals called odontocetes (toothed whales, including dolphins 1 Gustave L. Levy Place and porpoises) can also use echolocation sounds for navigation and prey track- Mail Box 1007 ing. In all cases, mammals use pneumatic (air-driven) mechanisms to generate New York, New York these sounds, but the sources and transmission pathways differ depending upon 10029-6574 whether sounds are emitted into air or water. USA Terrestrial Mammals Email: The voice box, or larynx, is the organ of vocalization used by most terrestrial mam- [email protected] mals. It initially evolved from the protective anatomy used to keep water out of a buoyancy organ in fish (the swim bladder). The main function of the larynx remains protection, only now it prevents incursions of foreign material into the “windpipe” (trachea) and lungs of mammals. The entrance of the larynx is sealed by a pair of vocal “cords” (vocal folds). In ad- dition, there are tall cartilages (epiglottic and corniculate) that act as splashguards to deflect food and water away from the opening. These cartilages overlap in front with the soft palate and behind with the posterior wall of the airspace (pharyn- geal wall) to interlock the larynx with the rear of the nasal cavity (Figure 1). -

NERVOUS SYSTEM : LEC / 8 Physiology of Nerves and Muscles the Nervous System Is One of the Most Complicated System of the Body in Both Structure and Function
Medical physiology Lecturer Second Stage Hiba Hazim Saleh NERVOUS SYSTEM : LEC / 8 Physiology of nerves and muscles The nervous system is one of the most complicated system of the body in both structure and function. It senses physical and chemical changes in the internal and external environment, processes them, and then responds to maintain homeostasis. Voluntary activities, such as walking and talking, and involuntary activities, such as digestion and circulation, are coordinates, regulated, and integrated by the nervous system. The entire neural network of the body relies on the transmission of nervous impulses. Nervous impulses are electrochemical stimuli that travel from cell to cell as they send information from one area of the body to another. The speed at which this occurs is almost instantaneous, thus providing an immediate response change. (Figure -1. ) Muscles Muscle tissue is composed of contractile cells or fibers that provide movement of an organ or body part. Muscles contribute to posture, produce body heat, and act as a protective covering for internal organs. Muscles make up the bulk of the body. They have the ability to be excited by a stimulus, contract, relax, and return to their original size and shape. Whether muscles are attached to bones or to internal organs and blood vessels, their primary responsibility is movement. Apparent motion provided by muscles include walking and talking. Less apparent motion include the passage and elimination of food through the digestive system, propulsion of blood through the arteries, and contraction of the bladder to eliminate urine. (Figure -2 .) Figure -1 Figure-2 Never Cell (Neuron): Nerve Cell: Is a basic unit of nervous system. -

The Human Brain Miguel Marín-Padilla
The Human Brain Miguel Marín-Padilla The Human Brain Prenatal Development and Structure Author Miguel Marín-Padilla Timberlea Circle 2447 55125 Woodbury, MN USA [email protected] ISBN 978-3-642-14723-4 e-ISBN 978-3-642-14724-1 DOI 10.1007/978-3-642-14724-1 Springer Heidelberg Dordrecht London New York Library of Congress Control Number: 2010936628 © Springer-Verlag Berlin Heidelberg 2011 This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilm or in any other way, and storage in data banks. Duplication of this publication or parts thereof is permitted only under the provisions of the German Copyright Law of September 9, 1965, in its current version, and permission for use must always be obtained from Springer. Violations are liable to prosecution under the German Copyright Law. The use of general descriptive names, registered names, trademarks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. Product liability: The publishers cannot guarantee the accuracy of any information about dosage and appli- cation contained in this book. In every individual case the user must check such information by consulting the relevant literature. Cover design: eStudioCalamar, Figueres/Berlin Printed on acid-free paper Springer is part of Springer Science+Business Media (www.springer.com) I dedicated this monograph to the memory of my dear Teresa, spouse, mother of my children, friend and life-long companion, since student years.