Doctoral Dissertation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
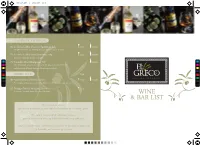
Wine List.Pdf 1 27/11/2017 11:42
Wine List.pdf 1 27/11/2017 11:42 CHAMPAGNE & SPARKLING 23. Le Dolci Colline Prosecco Spumante, Italy £5.95 £23.95 Delightfully fruity easy drinking Prosecco with a lovely clean finish 24. Le Dolci Colline Rose Spumante, Italy £5.95 £23.95 For those whose fizz just has to be pint C 25. Gremillet Brut Champagne NV £39.95 M This Champagne shows a fine mousse in the glass and some richer fruit Y and classic baked biscuit flavours with a crisp citrus finish. CM MY DESSERT WINE 75ml CY CMY 26. Mavrodaphne of Patras, Greece £5.50 £24.95 An exceptional deep sweet velvety fortified wine. The perfect end to any meal K 27. Bodegas Pinord, Moscatel, Spain £5.50 A luscious, honeyed dessert wine from Penedes. WINE & BAR LIST Not sure which wine..... you are very welcome to try a sample of any wine we sell by the glass. Not sure if you can finish a bo�le of wine..... you are welcome to take any unfinished bo�le away with you. Love a particular wine.....all wines are available to purchase to take away by the bo�le and to order by the case. Wine List.pdf 2 27/11/2017 11:42 WORLD WINES 175ml 250ml Bo�le HOUSE 1. White - Cape Heights, Sauvignon Blanc, South Africa £4.50 £5.90 £16.95 This Sauvignon from the Western Cape is zippy, racy and very pleasing. 2. Red - Cape Heights, Merlot, South Africa £4.50 £5.90 £16.95 Bright, juicy, plump and sensuous Merlot. GREEK WINES 3. -

Yiannis-Karakasis-Mw
Zambartas Winery in Cyprus: Wines of purity and character http://www.karakasis.mw/print/87 15 OCTOBER 2016 During my last year's, first visit to Cyprus I had the chance to taste numerous wines, from mineral Xynisteris and richer Promara to structured reds from Maratheftiko and Yiannoudi. You can read my first impressions and my tasting notes here. From what I have seen and tasted so far it is clear to me that Cyprus Wine Industry undergoes a major transformation. As Demetri Walters MW describes in his excellent article Cypriot wine: Going Native [1] the industry is changing its skin very rapidly shifting more attention to indigenous varieties. Work in progress maybe the best way to describe the current wine scene which is at crossroads. 1 of 3 1/11/2016 9:16 πμ Zambartas Winery in Cyprus: Wines of purity and character http://www.karakasis.mw/print/87 One of the pioneer Cypriot wineries is Zambartas [2]family winery whose wines I did not had the chance to taste while in Cyprus but winemaker Marcos Zambartas was kind enough to provide me with more food for thought, sending me samples including a single vineyard Xynisteri. The winery was established in 2006 by the late Akis Zambartas a visionary man who during his career as oenologist in KEO, re-discovered 12 native Cypriot grape varieties, which he recorded, planted and vinified. Among them the most important were: Maratheftiko, Lefkada, Promara, Spourtiko and Yiannoudi. Now the legacy of Akis Zambartas is continued through his son Marcos, a winemaker trained in Australia who runs the winery together with his wife Marleen. -

Wine List 2020
CHAMPAGNE & SPARKLING 23. Le Dolci Colline Prosecco Spumante, Italy £6.25 £24.95 Delightfully fruity easy drinking Prosecco with a lovely clean finish 24. Le Dolci Colline Rose Spumante, Italy £6.25 £24.95 For those whose fizz just has to be pint 25. Gremillet Brut Champagne NV £39.95 This Champagne shows a fine mousse in the glass and some richer fruit and classic baked biscuit flavours with a crisp citrus finish. DESSERT WINE 75ml 26. Mavrodaphne of Patras, Greece £5.50 £24.95 An exceptional deep sweet velvety fortified wine. The perfect end to any meal 27. Bodegas Pinord, Moscatel, Spain £5.50 A luscious, honeyed dessert wine from Penedes. WINE & BAR LIST Not sure which wine..... you are very welcome to try a sample of any wine we sell by the glass. Not sure if you can finish a bo�le of wine..... you are welcome to take any unfinished bo�le away with you. Love a particular wine.....all wines are available to purchase to take away by the bo�le and to order by the case. WORLD WINES 175ml 250ml Bo�le HOUSE 1. White - Cape Heights, Sauvignon Blanc, South Africa £4.75 £6.20 £18.95 This Sauvignon from the Western Cape is zippy, racy and very pleasing. 2. Red - Cape Heights, Merlot, South Africa £4.75 £6.20 £18.95 Bright, juicy, plump and sensuous Merlot. GREEK WINES 3. Rose - Burlesque, White Zinfandel, USA £4.75 £6.20 £18.95 Greece now produces some exceptionally good wines....why not rekindle those memories of long lazy days in A wine showing a bright fruit packed strawberry jam palate! the Mediterranean and try one .. -

Steven Shapin. the Tastes of Wine
n.s., 51 (3/2012), anno LII wineworld. new essays on wine, taste, philosophy and aesthetics advisory editor Nicola Perullo wineworld Nicola Perullo, Wineworld: Tasting, making, drinking, being 3 Steven Shapin, The tastes of wine: Towards a cultural history 49 Cain Todd, Expression and objectivity in the case of wine: Defending the aesthetic terroir of tastes and smells 95 Ole Martin Skilleås, Douglas Burnham, Patterns of attention: “Project” and the phenomenology of aesthetic perception 117 Kevin Sweeney, Structure in wine 137 Giampaolo Gravina, A matter of taste. The semi-serious musings of a wine taster on the contentious prospects of professional tasting 149 Gabriele Tomasi, On wines as works of art 155 Andrea Borghini, On being the same wine 175 varia Felice Cimatti, Quel dolore che non deve sapersi. Il linguaggio e il problema dell’esperienza estetica 193 recensioni Marcella Tarozzi Goldsmith, Il problema della percezione nella filosofia di Nietzsche, di Tiziana Andina 215 Emanuele Crescimanno, La scrittura delle immagini. Letteratura e cultura visuale, di Michele Cometa 221 Steven Shapin THE TASTES OF WINE: TOWARDS A CULTURAL HISTORY Abstract How have people talked about the organoleptic characteristics of wines? How and why have descriptive and evaluative vocabularies changed over time? The essay shows that these vocabularies have shifted from the spare to the elaborate, from medical im- plications to aesthetic analyses, from a leading concern with “goodness” (authenticity, soundness) to interest in the analytic description of component flavors and odors. The causes of these changes are various: one involves the importance, and eventual disap- pearance, of a traditional physiological framework for appreciating the powers and qualities of different sorts of aliment, including wines; another concerns the develop- ment of chemical sciences concerned with flavor components; and still another flows from changing social and economic circumstances in which wine was consumed and the functions served by languages of connoisseurship. -

A Conceptual Framework Towards Succession Effectiveness in Family Wineries
A CONCEPTUAL FRAMEWORK TOWARDS SUCCESSION EFFECTIVENESS IN FAMILY WINERIES THE “WINESUCCESS” CONCEPTUAL FRAMEWORK THOUKIS GEORGIOU A thesis submitted to The University of Gloucestershire In accordance with the requirements of the degree of Doctor of Philosophy In the Business School February, 2018 ABSTRACT Succesion effectiveness in family wineries is considered critical for the incumbents and the owning families looking forward to assure winery success and transgenerational continuity, as this endeavour requires substantial commitment, social skills, financial health, and idiosyncratic considerations that are more often than not unstable. Therefore, this thesis provides a platform of critical reflection and theoretical development upon the findings of a doctoral research on the topic of effective succession in family wineries for bringing further and closer theory and professional practice. Systematic literature review of the best available sources of knowledge served as a starting point. It was shown that there is a plethora of academic research on effective family business succession that makes available useful insights into this important process. The review has identified the major theories, models, and frameworks, and provided information on different factors and variables that are believed appropriate to foster succession process further. However, the review findings are often fragmented and subjective which makes it difficult to draw valid conclusions that can be representative for family wineries. Moreover, the review revealed certain gaps and uncertainties in the research that this thesis has aimed to bridge, and allowed the development of a preliminary conceptual framework (version one) with the testable research hypotheses. A primary research that followed in the organizational context of Cypriot family wineries made use of both quantitative and qualitative approaches, at a greater and lesser extent, respectively. -
Wine List Jul20-Web
WORLD WINES 175ml 250ml Bo�le HOUSE 1. White - Cape Heights, Sauvignon Blanc, South Africa £4.75 £6.20 £18.95 This Sauvignon from the Western Cape is zippy, racy and very pleasing. 2. Red - Cape Heights, Merlot, South Africa £4.75 £6.20 £18.95 Bright, juicy, plump and sensuous Merlot. 3. Rose - Burlesque, White Zinfandel, USA £4.75 £6.20 £18.95 A wine showing a bright fruit packed strawberry jam palate! WHITE 4. Mirabello, Pinot Grigio, Italy £4.95 £6.50 £19.95 Many PG’s lack flavour but not this one! Clean and dry with some notable fruit on the finish. 5. Soldiers Block, Chardonnay, Australia £5.25 £ 6.95 £21.95 This vibrant Chardonnay exhibits lifted fruit character, textural mouth-feel, a bright palate and a long foodie finish. 6. Adobe Viognier (organic), Chile £23.95 Dry with rounded slightly spicy fruit. 7. Moko Black, Sauvignon Blanc, New Zealand £25.95 Pungent gooseberry and grass with a touch of elderflower layered into the flavour palate. 8. Chablis, Domaine De La Mo�e, France £35.95 Classic Chablis with that lovely steeliness and a good long finish. RED 9. Soldier’s Block, Shiraz, Australia £ 5.25 £6.95 £21.95 A bright style of Shiraz with a silky mouthfeel with a touch of vanilla, as well as some freshness and an elegant finish. 10. Vega del Rayo Seleccionada, Rioja, Spain £6.25 £7.75 £24.95 This Rioja combines great balance, approachable fruit, spice and a smoky, spicy dimension from six months in oak. 11. Los Haroldos (oak aged), Malbec, Argentina £27.95 This Malbec shows ripe fruits with a subtle integration of oak from six months oak ageing in French and American oak. -
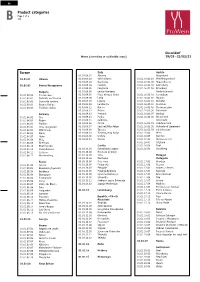
B Product Categories
2021_PROWEIN_B_EN.qxp_2021_PROWEIN_B_EN 08.10.20 12:45 Seite 1 GB Product categories Page 1 of 4 B 1.0 Düsseldorf Wines (according to cultivable areas) 19/03–23/03/21 Europe Italy Austria 01.01.09.01 Abruzzo Burgenland 01.01.01 Albania 01.01.09.02 Valle d'Aosta 01.01.16.01.01 Mittelburgenland 01.01.09.03 Basilicata 01.01.16.01.02 Neusiedlersee 01.01.02 Bosnia-Herzegowina 01.01.09.04 Calabria 01.01.16.01.03 Leithaberg 01.01.09.05 Campania 01.01.16.01.04 Eisenberg Bulgaria 01.01.09.06 Emilia-Romagna Niederösterreich 01.01.03.01 Cherno more 01.01.09.07 Friuli Venezia-Giulia 01.01.16.02.01 Carnuntum 01.01.03.02 Dolinata na Strouma 01.01.09.08 Lazio 01.01.16.02.02 Wagram 01.01.03.03 Dunavska ravnina 01.01.09.09 Liguria 01.01.16.02.03 Kamptal 01.01.03.04 Rozova Dolina 01.01.09.10 Lombardia 01.01.16.02.04 Kremstal 01.01.03.05 Trakiiska nizina 01.01.09.11 Marche 01.01.16.02.05 Thermenregion 01.01.09.12 Molise 01.01.16.02.06 Traisental Germany 01.01.09.13 Piemont 01.01.16.02.07 Wachau 01.01.04.01 Ahr 01.01.09.14 Puglia 01.01.16.02.08 Weinviertel 01.01.04.02 Baden 01.01.09.15 Sardegna Steiermark 01.01.04.03 Franken 01.01.09.16 Sicilia 01.01.16.03.01 Südsteiermark 01.01.04.04 Hess. -

Review of the Wine Industry in Cyprus and Europe
Advanced systems for the enhancement of the environmental performance of WINEries in Cyprus (WINEC) Deliverable 1 Review of the wine industry in Cyprus and in Europe Ms Lida Ioannou Ms Margarita Vatyliotou Dr. Despo Fatta‐Kassinos, Head of GAIA Second Edition Nicosia, July 2010 Summary The present study deals with the investigation of the wine industry in Europe and especially in Cyprus. In specific wine production and consumption, the exports and imports of wine and the percentage Europe possesses in the world wine production are examined. Moreover, the first six wine producing European countries (Italy, France, Spain, Germany, Portugal and Greece) are analytically described in terms of their grape varieties, wine produced and area of vineyards. Information about the geographical distribution of wineries, the wine producing methods used all over Europe as well as figures on the grapes and wine production is provided. With regard to the wine industry in Cyprus, the wine producing regions of the Republic, the main wine producers as well as the indigenous and imported grape varieties are determined. Finally through this study the red, white, rose and sparkling wine production methodologies are extensively reviewed. In terms of wine industry in Europe, it can be said that France and Italy are the largest wine producer countries in the world, while Europe dominates in the global wine production possessing the 69%. Spain which is the third largest wine producer country in the world, has over than 1.17 million hectares planted, making it the most widely planted wine producing nation. The sixth largest wine producer European country is Greece, while Cyprus is the fourteenth. -

C 304 Official Journal
ISSN 1977-091X Official Journal C 304 of the European Union Volume 55 English edition Information and Notices 9 October 2012 Notice No Contents Page II Information INFORMATION FROM EUROPEAN UNION INSTITUTIONS, BODIES, OFFICES AND AGENCIES European Commission 2012/C 304/01 Authorisation for State aid pursuant to Articles 107 and 108 of the TFEU — Cases where the Commission raises no objections ( 1) . 1 2012/C 304/02 Authorisation for State aid pursuant to Articles 107 and 108 of the TFEU — Cases where the Commission raises no objections ( 2) . 2 2012/C 304/03 Decisions in the context of the monitoring of the implementation of decisions regarding restructuring and liquidation aid for financial institutions ( 2) . 3 2012/C 304/04 Non-opposition to a notified concentration (Case COMP/M.6693 — Tech Data/BEL) ( 2 ) . 4 2012/C 304/05 Non-opposition to a notified concentration (Case COMP/M.6599 — Comsa Rail Transport/Naviland Cargo/Grupo Logistico SESE/Target) ( 2 ) . 4 Price: ( 1 ) Text with EEA relevance, except for products falling under Annex I to the Treaty 2 EN EUR 3 ( ) Text with EEA relevance (Continued overleaf) Notice No Contents (continued) Page IV Notices NOTICES FROM EUROPEAN UNION INSTITUTIONS, BODIES, OFFICES AND AGENCIES European Commission 2012/C 304/06 Euro exchange rates . 5 V Announcements PROCEDURES RELATING TO THE IMPLEMENTATION OF COMPETITION POLICY European Commission 2012/C 304/07 Prior notification of a concentration (Case COMP/M.6541 — Glencore/Xstrata) ( 1) . 6 2012/C 304/08 Prior notification of a concentration (Case COMP/M.6677 — MOL/KMG EP/JV) — Candidate case for simplified procedure ( 1 ) . -

Today, Around 50 Boutique Wineries, Many the Pet
winetravel WORDS ELISABETH KING CYPRUS - LAND OF LOVE AND WINE TO BE honest, Cyprus had never been I stayed in the Greek part of the island, sea views truly deserve that over-used high on my travel wish list. Too many officially called the Republic of Cyprus. adjective - stunning. British package tourists (1.5 million a The most intriguing place to explore Limassol, like Paphos, is a major resort year) looking for a cheap lifestyle in the its tangled history is Nicosia, which town and the biggest port on the island sun and too much historical baggage enjoys the dubious claim of being the where history lurks amid the modern over the partition of the island always put only divided capital city in the world. malls, plazas, hotels and office blocks. me off. But these prejudices faded fast There have been several outbreaks of The oldest part of town dates back to the as I took a dip near Petra tou Romiu, a harmony, as the locals put it, since 2003 13th century and there’s always a crowd craggy rock off the southern coast of the and there’s now six crossings which massed at the Byzantine castle. There’s a island, where legend has it that Aphrodite allow tourists to explore the Greek and lot more people congregating in the bars first emerged from the sea. Turkish sections. and clubs, however, where the real action If the Greek goddess of love reprised The Greek part of the city, with Venetian starts after 2am. the same foam-flecked event today, ramparts and designer shops where Spring and autumn are the optimum she would make straight for the nearby you can buy up big in euros, is far ritzier times to visit Cyprus unless you prefer Columbia Beach Resort overlooking than the “other side”. -

NEO 2009.05 May.Cdr
:: MAY 2009 $2.95 Special Cypriot American Issue Cyprus Ascendant Cyprus ready for business Cyprus wines launch new campaign Cyprus Exhibition in Chicago CYPRUS FEDERATION NATIONAL CONVENTION Human Rights Champion US Senator RobertRobert MenendezMenendez New York City Greek Independence Day Parade L100 New Generation Leader Justin Bozonelis Anna Vissi in Hollywood! 6 12 Sen. Bob Menendez “Our decisions on the major 33 issues are going to write the type of century this will be for America and for the world” 20 23 18 32 Cyprus – US Chamber Aris Melissaratos 37 of Commerce Merit honored by his fellow Award Cephalonians 19 34 Exhibition on the Cyprus Federation Movement for a Greek pride marched The New Generation of America: A force United Cyprus in along Fifth Avenue of Leaders to reckon with Chicago 19 8 22 24 38 Cyprus: Ready for Business L100 Partners kick off Cultural or Religious more than ever Membership Campaign Identity 10 16 28 33 Cyprus Wine PSEKA gathers Association launches Cypriots in DC for its Capital Link Forum on Anna Vissi in US campaign annual conference International Shipping Hollywood Cyprus and its champions he very best kind of friend that Cyprus has is U.S. :: magazine Senator Robert Menendez of New Jersey, himself Tthe son of immigrants, who as my colleague Demetrios Rhompotis describes it, one day “met his constituent and neighbor Tasos Zambas, who challenged Editor in Chief: him to help him visit his house in the occupied area Dimitri C. Michalakis Cyprus after 30 years. Menendez not only took the [email protected] -

The Cyprus Wine Revolution | Articles
● Written by Jancis Robinson 3 May 2017 The Cyprus wine revolution To complement Dr José Vouillamoz's fascinating report on discovering indigenous grape varieties on the Mediterranean island of Cyprus, I'm publishing my notes on a presentation on the recent Cypriot wine revolution made last October by Dr Caroline Gilby MW, one of the five finalists in our recent wine writing competition. Her lecture and the tasting that followed it (see my 42 tasting notes below) took place in Vintners' Hall in London, where the then king of Cyprus was one of the five kings entertained to a feast by the vintners and aldermen in 1363. (For much of its early history, the Wine & Spirit Education Trust was based next to Vintners' Hall in a building known as Five Kings House.) Last time I studied the wine scene on Cyprus in any detail, it was dominated by four big wineries located on the coast, far from the vineyards but convenient for the tankers that used to ship out vast quantities of a fortified wine of dubious quality, known, until the Spanish had their protective way, as 'Cyprus sherry'. The island's wine industry is still very much work in progress but the big transformation has been that the island now has 60-odd much smaller wineries typically processing 3,000 to 30,000 tonnes of grapes a year, run by well-travelled, well-trained young specialists. More crucially, they are located close to vineyards up in the hills in the interior of the island such as those of Tsiakkas at Pitsilia above, photographed by Caroline.