Cataract Surgery and Dry Eye
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Visual Outcomes of Combined Cataract Surgery and Minimally Invasive Glaucoma Surgery
1422 REVIEW/UPDATE Visual outcomes of combined cataract surgery and minimally invasive glaucoma surgery Steven R. Sarkisian Jr, MD, Nathan Radcliffe, MD, Paul Harasymowycz, MD, Steven Vold, MD, Thomas Patrianakos, MD, Amy Zhang, MD, Leon Herndon, MD, Jacob Brubaker, MD, Marlene Moster, MD, Brian Francis, MD, for the ASCRS Glaucoma Clinical Committee Minimally invasive glaucoma surgery (MIGS) has become a reliable on visual outcomes based on the literature and the experience of standard of care for the treatment of glaucoma when combined the ASCRS Glaucoma Clinical Committee. with cataract surgery. This review describes the MIGS procedures J Cataract Refract Surg 2020; 46:1422–1432 Copyright © 2020 Published currently combined with and without cataract surgery with a focus by Wolters Kluwer on behalf of ASCRS and ESCRS inimally invasive (sometimes referred to as mi- and thereby lower IOP. The endoscope consists of a fiber- croinvasive) glaucoma surgery (MIGS) is a pro- optic camera, light source, and laser aiming beam with an Mcedure that lowers intraocular pressure (IOP) 832 nm diode laser. The endoscope probe is introduced into without significantly altering the tissue, allows for rapid the globe via a limbal corneal or pars plana incision. The visual recovery, is moderately effective, and can be com- anterior approach requires inflation of the ciliary sulcus with bined with cataract surgery in a safe and efficient manner.1,2 an ophthalmic viscosurgical device, whereas the posterior This is in contrast to more conventional glaucoma surgery approach uses a pars plana or anterior chamber irrigation (eg, trabeculectomy or large glaucoma drainage device port. Although the anterior approach can be used in a phakic implantation), which requires conjunctival and scleral eye, it is typically performed with cataract extraction as a incisions as well as suturing. -

Ocular Surface Disease: Supplement April 2018 Accurately Diagnose & Effectively Treat Your Surgical Patients
Ocular Surface Disease: Supplement April 2018 Accurately Diagnose & Effectively Treat Your Surgical Patients Supported by an unrestricted educational grant from Ocular Surface Disease: Accurately Diagnose & Effectively Treat Your Surgical Patients Prevalence of Ocular Surface Disease and Its Impact on Surgical Outcomes Accurate diagnosis of dry eye disease is critical before cataract or refractive surgery By Elisabeth M. Messmer, MD ry eye is a common disease, but it may remain EPIDEMIOLOGY OF DRY EYE SYNDROME undetected. If it is not treated before cataract or 1-4 refractive surgery, patients may have suboptimal visual AFTER CATARACT SURGERY outcomes from their procedures. D l Very limited data available, mostly small descriptive/ IMPACT ON CATARACT SURGERY non-randomised studies There are a number of triggering factors for dry eye (Figure 1). l 10-20% of patients: DED induced or worsened after Cataract surgery worsens or causes dry eye in approximately uncomplicated cataract surgery 10% to 20% of patients (Figure 2).1-4 l In all studies: Signs and symptoms of dry eye In a study of 136 patients with a mean age of 71 years who increase after surgery were having cataract surgery, 22% had a prior diagnosis of dry eye that was not treated.5 Thirty-one percent complained l In most studies: gradual improvement of signs and of stinging, burning or other symptoms of dry eye when asked symptoms of dry eye within 3 months about their symptoms, and 41% reported a foreign body l In some studies: signs and symptoms persist > 3 months sensation. When the patients were examined, 77% had corneal staining and 50% had central staining. -

CAUSES, COMPLICATIONS &TREATMENT of A“RED EYE”
CAUSES, COMPLICATIONS & TREATMENT of a “RED EYE” 8 Most cases of “red eye” seen in general practice are likely to be conjunctivitis or a superficial corneal injury, however, red eye can also indicate a serious eye condition such as acute angle glaucoma, iritis, keratitis or scleritis. Features such as significant pain, photophobia, reduced visual acuity and a unilateral presentation are “red flags” that a sight-threatening condition may be present. In the absence of specialised eye examination equipment, such as a slit lamp, General Practitioners must rely on identifying these key features to know which patients require referral to an Ophthalmologist for further assessment. Is it conjunctivitis or is it something more Iritis is also known as anterior uveitis; posterior uveitis is serious? inflammation of the choroid (choroiditis). Complications include glaucoma, cataract and macular oedema. The most likely cause of a red eye in patients who present to 4. Scleritis is inflammation of the sclera. This is a very rare general practice is conjunctivitis. However, red eye can also be presentation, usually associated with autoimmune a feature of a more serious eye condition, in which a delay in disease, e.g. rheumatoid arthritis. treatment due to a missed diagnosis can result in permanent 5. Penetrating eye injury or embedded foreign body; red visual loss. In addition, the inappropriate use of antibacterial eye is not always a feature topical eye preparations contributes to antimicrobial 6. Acid or alkali burn to the eye resistance. The patient history will usually identify a penetrating eye injury Most general practice clinics will not have access to specialised or chemical burn to the eye, but further assessment may be equipment for eye examination, e.g. -

Contacts Vs. Iols for Congenital Cataract
in Review News commentary and perspectives Contacts vs. IOLs for Congenital Cataract he verdict is in on the issue of optical correction in children who undergo unilateral cataract surgery before age 7 months: Aphakia, corrected with a contact lens, is a better option than an T CONTACT LENS PATIENT. Dr. Lambert examines a 6-year-old intraocular lens (IOL) for 55 others who received an aphakic girl in the IATS trial. This child was prescribed a most of these babies. IOL implant (median VA in contact lens in one eye at 1 month of age and could insert “Primary IOL implan- both groups, 0.90 logMAR her own contact lens by the age 4 years. tation should be reserved [20/159]). for those infants where, in More complications. pillary membranes occurred one normal eye. But the the opinion of the surgeon, However, a significantly 10 times more often in the thing about children is that the cost and handling of greater number of the pseu- pseudophakic eyes. they’re going to live for a a contact lens would be so dophakic eyes required one Scott R. Lambert, MD, very long time, and it is burdensome as to result in or more additional intra- a professor of ophthalmol- important for them to have significant periods of uncor- operative procedures over ogy at Emory University in the best possible visual acu- rected aphakia,” stated the the course of the study (41 Atlanta and the lead inves- ity in their problem eye,” investigators in the Infant patients compared with tigator in the trial, credited he said, particularly in case Aphakia Treatment Study.1 12 in the aphakic group; advocacy by the pediatric anything should happen to Comparable VA. -

Treatment of Stable Keratoconus by Cataract Surgery with Toric IOL Implantation
10.5005/jp-journals-10025-1024 JaimeCASE Levy REPORT et al Treatment of Stable Keratoconus by Cataract Surgery with Toric IOL Implantation Jaime Levy, Anry Pitchkhadze, Tova Lifshitz ABSTRACT implantation in the right eye. On presentation, uncorrected We present the case of a 73-year-old patient who underwent visual acuity (UCVA) was 6/60 OU. Refraction was –0.75 successful phacoemulsification and toric intraocular lens (IOL) –5.0 × 65° OD and –3.25 –4.0 × 98° OS. Nuclear sclerosis implantation to correct high stable astigmatism due to and posterior subcapsular cataract +2 was observed in the keratoconus and cataract. Preoperative refraction was –3.25 – left eye. The posterior segments were unremarkable. 4.0 × 98°. A toric IOL (Acrysof SN60T6) with a spherical power of 16.5 D and a cylinder power of 3.75 D at the IOL plane and Corneal topography performed with Orbscan (Bausch 2.57 D at the corneal plane was implanted and aligned at an and Lomb, Rochester, NY) showed central thinning of 457 axis of 0°. Uncorrected visual acuity improved from 6/60 to microns and positive islands of elevation typical for 6/10. Postoperative best corrected visual acuity was 6/6, 6 months after the operation. In conclusion, phacoemulsification keratoconus in the right eye (Fig. 1). In the left eye a less with toric IOL implantation can be performed in eyes with pronounced inferior cone was observed (Fig. 2), without keratoconus and cataract. any area of significant thinning near the limbus typical for Keywords: Intraocular lens, Toric IOL, Keratoconus, Cataract pellucid marginal degeneration.2 Keratometry (K)-values surgery. -

Incidence of Posterior Vitreous Detachment After Cataract Surgery
ARTICLE Incidence of posterior vitreous detachment after cataract surgery Alireza Mirshahi, MD, FEBO, Fabian Hoehn, MD, FEBO, Katrin Lorenz, MD, Lars-Olof Hattenbach, MD PURPOSE: To report the incidence of posterior vitreous detachment (PVD) after uneventful state- of-the-art small-incision phacoemulsification with implantation of a posterior chamber intraocular lens (PC IOL). SETTING: Department of Ophthalmology, Ludwigshafen Hospital, Ludwigshafen, Germany. METHODS: This prospective study evaluated the vitreous status of eyes by biomicroscopic exam- ination, indirect binocular ophthalmoscopy, and B-scan ultrasonography before planned cataract surgery. Patients with the posterior vitreous attached were included for follow-up and examined 1 week, 1 month, and 1 year after uneventful phacoemulsification with PC IOL implantation. The preoperative prevalence and postoperative incidence of PVD were determined by ultrasonography. RESULTS: The study included 188 eyes of 188 patients (131 women, 57 men) with a mean age of 77.2 years. The mean spherical equivalent was À0.78 diopter (D) (range À8.75 to C6.25 D) and the mean axial length (AL), 23.22 mm (range 20.50 to 26.04 mm). Preoperatively, 130 eyes (69.1%) had PVD and 58 eyes (30.9%) had no PVD. Postoperatively, 12 eyes (20.7%) developed PVD at 1 week, 18 eyes (31%) at 1 month, and 4 eyes (6.9%) at 1 year. The vitreous body remained at- tached to the retina in 24 eyes (41.4%) 1 year after surgery. No preoperatively measured parameter (eg, age, refraction, AL, effective phacoemulsification time) was predictive of the occurrence of PVD after cataract surgery. CONCLUSION: The occurrence of PVD after modern cataract surgery was frequent in cases in which the posterior hyaloid was attached to the retinal surface preoperatively. -

Florida Board of Medicine and Florida Board Of
FLORIDA BOARD OF MEDICINE AND FLORIDA BOARD OF OSTEOPATHIC MEDICINE APPROVED INFORMED CONSENT FORM FOR CATARACT OPERATION WITH OR WITHOUT IMPLANTATION OF INTRAOCULAR LENS DOES THE PATIENT NEED OR WANT A TRANSLATOR, INTERPRETOR OR READER? YES _____ NO_____ TO THE PATIENT: You have the right, as a patient, to be informed about your cataract condition and the recommended surgical procedure to be used, so that you may make the decision whether or not to undergo the cataract surgery, after knowing the risks, possible complications, and alternatives involved. This disclosure is not meant to scare or alarm you; it is simply an effort to make you better informed so that you may give or withhold your consent to cataract surgery and should reflect the information provided by your eye surgeon. If you have any questions or do not understand the information, please discuss the procedure with your eye surgeon prior to signing. WHAT IS A CATARACT, AND HOW IS IT TREATED? The lens in the eye can become cloudy and hard, a condition known as a cataract. Cataracts can develop from normal aging, from an eye injury, various medical conditions or if you have taken certain medications such as steroids. Cataracts may cause blurred vision, dulled vision, sensitivity to light and glare, and/or ghost images. If the cataract changes vision so much that it interferes with your daily life, the cataract may need to be removed to try to improve your vision. Surgery is the only way to remove a cataract. You can decide to postpone surgery or not to have the cataract removed. -
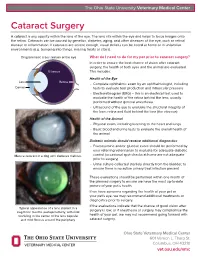
Cataract Surgery a Cataract Is Any Opacity Within the Lens of the Eye
The Ohio State University Veterinary Medical Center Cataract Surgery A cataract is any opacity within the lens of the eye. The lens sits within the eye and helps to focus images onto the retina. Cataracts can be caused by genetics, diabetes, aging, and other diseases of the eye, such as retinal disease or inflammation. If cataracts are severe enough, visual deficits can be noted at home or in unfamiliar environments (e.g. bumping into things, missing treats or stairs). Diagrammatic cross section of the eye What do I need to do for my pet prior to cataract surgery? In order to ensure the best chance of vision after cataract surgery, the health of both eyes and the animal are evaluated. Vitreous This includes: Health of the Eye Lens Retina • Complete ophthalmic exam by an ophthalmologist, including Cornea tests to evaluate tear production and intraocular pressure • Electroretinogram (ERG) – this is an electrical test used to evaluate the health of the retina behind the lens, usually performed without general anesthesia • Ultrasound of the eye to evaluate the structural integrity of the lens, retina and fluid behind the lens (the vitreous) Health of the Animal • Physical exam, including listening to the heart and lungs • Basic blood and urine tests to evaluate the overall health of the animal Diabetic animals should receive additional diagnostics • Fructosamine and/or glucose curve should be performed by your referring veterinarian to evaluate for adequate diabetic Mature cataract in a dog with diabetes mellitus. control (occasional spot-checks at home are not adequate prior to surgery) • Urine culture collected sterilely directly from the bladder, to ensure there is no active urinary tract infection present These evaluations should be performed within one month of the planned surgery to ensure we have the most up-to-date picture of your pet’s health. -

Tips and Techniques for Cataract Surgery in Glaucoma Patients
Tips and Techniques for Cataract Surgery in Glaucoma Patients Joey Yen-Cheng Hsia, MD Assistant Professor of Ophthalmology Glaucoma Service University of California, San Francisco No Financial Disclosures Introduction • Visually significant cataract often co-exist with glaucoma in the elderly population. • Glaucoma incisional surgery can lead to accelerated cataract formation. • Glaucoma patients are at risk for perioperative complications • Set realistic expectation preoperatively Preoperative Evaluation – Is the cataract or glaucoma causing the decreased vision? – PAP or PAM – Set realistic expectation – Is the IOP at target? – Role of combined surgery? – No. of medications – Anticoagulation – ⍺-1 blocker – Prior incisional surgeries Examination – angle grading, trab ostium - Prior incisional surgery - endothelial dysfunction – shallowing – dilation, prior LPI, iridectomy – PXE, phacodynesis – cupping, pallor, retinal pathology Postoperative IOP Spike • Note the of glaucoma – Foveal involving scotoma – At risk for progression with IOP spike : – Advanced glaucoma, IFIS, No. of gtts, long AXL, PXE • IOP spike occurs after surgery – Same day check up for high risk patients History of Trabeculectomy – Modify incisions accordingly – Avoid suction / fixation ring – High function bleb may lead to chemosis / chamber instability – Age<50, Preop IOP > 10, iris manipulation, postop IOP spike, and short interval time between trabeculectomy and cataract – Longer steroid +/- anti- metabolite Grover-Fellman spatula; Epislon History of Tube Shunt – -

History of Cataract Surgery
History of Cataract Surgery A cataract is a clouding of the normally clear lens of the eye and can be addressed through a procedure that removes the affected lens and replaces it with a manmade lens known as an intraocular lens or IOL.1 Associated with improvements in vision, overall health, and cognitive and emotional well-being,2 cataract surgery is a safe and effective procedure1 and is the most commonly performed surgery in the world.3 One of the oldest surgical procedures known, cataract surgery was first documented in the fifth century BC.4 1747 French ophthalmologist Jacques Daviel is credited 1998 Toric IOLs are rolled out to correct astigmatism. Toric with the first Extracapsular Cataract Extraction, a IOLs have different powers in different meridians of the technique that uses a small incision to remove the lens and lens to correct the asymmetric power of the eye that is minimize the wound.5,6 characteristic of astigmatism.10 1753 London surgeon Samuel Sharp performs the first 2004 Aspheric IOLs, which closely match the shape and optical Intracapsular Cataract Extraction, a technique that quality of the eye’s natural lens, are introduced for sharper uses a large incision to remove the entire natural vision — especially in low light conditions and when the lens and capsule.7 pupil is dilated.11 1949 Intraocular lenses, manmade lenses made of 2010 Femtosecond laser is cleared by the FDA for cataract polymethylmethacrylate, are introduced by Sir Harold surgery.12 The femtosecond laser replaces or supports Ridley in London.8 Previously, -

Cataract Surgery and Retinal Detachment: Cause and Effect? Br J Ophthalmol: First Published As 10.1136/Bjo.80.8.683 on 1 August 1996
British Journal of Ophthalmology 1996;80:683-684 683 Cataract surgery and retinal detachment: cause and effect? Br J Ophthalmol: first published as 10.1136/bjo.80.8.683 on 1 August 1996. Downloaded from Retinal detachment following cataract surgery is a serious Measures of effect, such as relative risk, provide some and potentially sight threatening event that will often assessment of the magnitude of an association between an necessitate further surgical intervention. Because of the exposure (cataract surgery) and the condition (retinal temporal sequence of events, any retinal detachment detachment), indicating the likelihood of developing the following cataract surgery is often assumed to be causally condition in the exposed group relative to those who are related to the cataract extraction. The evidence for this not exposed. The identification of a control group by Nor- relation has been based on the observed frequency of such regaard and colleagues permits this kind of assessment of events following cataract surgery, particularly the excess the risk of retinal detachment associated with cataract sur- frequency observed after intracapsular cataract extraction gery. Taking the standardised incidence ratios that are pre- (ICCE) compared with extracapsular cataract extraction sented in this study (as estimates of relative risk), it would (ECCE). All these observations relate to surgical practice appear that the risk 4 years after surgery, for the ECCE and at least a decade ago and are characterised by the absence IOL group, is over 4.4 times that of the control group. of a control group of patients who did not have cataract The relative risk indicates the strength of an aetiological surgery and their experience of retinal detachment for (or causal) association between cataract surgery and retinal comparison. -

Cataract Surgery Will Improve Your Vision
What is a Cataract? We all have a natural lens inside of our eyes. This lens helps to focus our vision. As our eyes age or other diseases affect the eyes, the natural lens becomes cloudy and disturbs good vision. This is called a cataract. The Initial Cataract Evaluation An initial evaluation is performed to determine if you have a cataract, and whether it can be safely removed. A full eye exam is performed during this visit, which involves dilating the eyes. Furthermore, we determine if cataract surgery will improve your vision. With cuts to Medicare and health insurance, we are now only allowed to perform the most basic diagnostic testing during this evaluation. In the past, we were able to perform these advanced preoperative tests during the initial cataract evaluation. The Preoperative Refractive Evaluation (PRE) After your initial evaluation, a PRE is scheduled on a separate day sometime shortly before your Cataract Surgery. During this evaluation, your eyes are typically not dilated and we perform a multitude of advanced diagnostic tests. The results of these tests are vital to the success of your cataract surgery. To schedule cataract surgery with Dr. McGarity, this evaluation is highly recommended. Cataract Surgery At our vision correction center, Dr. McGarity performs surgery using phacoemulsification. Using ultrasound technology, phacoemulsification gently dissolves and aspirates the cataract from the eye. Then a biocompatible, customized, prescription lens, known as an intraocular lens (IOL), is permanently inserted in your eye. The PRE is essential in providing the most accurate measurements to select the best IOL prescription for your eye.