The Genetic Basis of Human Brain Structure and Function
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Plasma Peptides of Alzheimer's Disease
Florentinus‑Mefailoski et al. Clin Proteom (2021) 18:17 https://doi.org/10.1186/s12014‑021‑09320‑2 Clinical Proteomics RESEARCH Open Access The plasma peptides of Alzheimer’s disease Angelique Florentinus‑Mefailoski1, Peter Bowden1, Philip Scheltens2, Joep Killestein3, Charlotte Teunissen4 and John G. Marshall1,5* Abstract Background: A practical strategy to discover proteins specifc to Alzheimer’s dementia (AD) may be to compare the plasma peptides and proteins from patients with dementia to normal controls and patients with neurological condi‑ tions like multiple sclerosis or other diseases. The aim was a proof of principle for a method to discover proteins and/ or peptides of plasma that show greater observation frequency and/or precursor intensity in AD. The endogenous tryptic peptides of Alzheimer’s were compared to normals, multiple sclerosis, ovarian cancer, breast cancer, female normal, sepsis, ICU Control, heart attack, along with their institution‑matched controls, and normal samples collected directly onto ice. Methods: Endogenous tryptic peptides were extracted from blinded, individual AD and control EDTA plasma sam‑ ples in a step gradient of acetonitrile for random and independent sampling by LC–ESI–MS/MS with a set of robust and sensitive linear quadrupole ion traps. The MS/MS spectra were ft to fully tryptic peptides within proteins identi‑ fed using the X!TANDEM algorithm. Observation frequency of the identifed proteins was counted using SEQUEST algorithm. The proteins with apparently increased observation frequency in AD versus AD Control were revealed graphically and subsequently tested by Chi Square analysis. The proteins specifc to AD plasma by Chi Square with FDR correction were analyzed by the STRING algorithm. -

Genome-Wide Transcriptome Analysis of Laminar Tissue During the Early Stages of Experimentally Induced Equine Laminitis
GENOME-WIDE TRANSCRIPTOME ANALYSIS OF LAMINAR TISSUE DURING THE EARLY STAGES OF EXPERIMENTALLY INDUCED EQUINE LAMINITIS A Dissertation by JIXIN WANG Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY December 2010 Major Subject: Biomedical Sciences GENOME-WIDE TRANSCRIPTOME ANALYSIS OF LAMINAR TISSUE DURING THE EARLY STAGES OF EXPERIMENTALLY INDUCED EQUINE LAMINITIS A Dissertation by JIXIN WANG Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Approved by: Chair of Committee, Bhanu P. Chowdhary Committee Members, Terje Raudsepp Paul B. Samollow Loren C. Skow Penny K. Riggs Head of Department, Evelyn Tiffany-Castiglioni December 2010 Major Subject: Biomedical Sciences iii ABSTRACT Genome-wide Transcriptome Analysis of Laminar Tissue During the Early Stages of Experimentally Induced Equine Laminitis. (December 2010) Jixin Wang, B.S., Tarim University of Agricultural Reclamation; M.S., South China Agricultural University; M.S., Texas A&M University Chair of Advisory Committee: Dr. Bhanu P. Chowdhary Equine laminitis is a debilitating disease that causes extreme sufferring in afflicted horses and often results in a lifetime of chronic pain. The exact sequence of pathophysiological events culminating in laminitis has not yet been characterized, and this is reflected in the lack of any consistently effective therapeutic strategy. For these reasons, we used a newly developed 21,000 element equine-specific whole-genome oligoarray to perform transcriptomic analysis on laminar tissue from horses with experimentally induced models of laminitis: carbohydrate overload (CHO), hyperinsulinaemia (HI), and oligofructose (OF). -

WO 2019/068007 Al Figure 2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2019/068007 Al 04 April 2019 (04.04.2019) W 1P O PCT (51) International Patent Classification: (72) Inventors; and C12N 15/10 (2006.01) C07K 16/28 (2006.01) (71) Applicants: GROSS, Gideon [EVIL]; IE-1-5 Address C12N 5/10 (2006.0 1) C12Q 1/6809 (20 18.0 1) M.P. Korazim, 1292200 Moshav Almagor (IL). GIBSON, C07K 14/705 (2006.01) A61P 35/00 (2006.01) Will [US/US]; c/o ImmPACT-Bio Ltd., 2 Ilian Ramon St., C07K 14/725 (2006.01) P.O. Box 4044, 7403635 Ness Ziona (TL). DAHARY, Dvir [EilL]; c/o ImmPACT-Bio Ltd., 2 Ilian Ramon St., P.O. (21) International Application Number: Box 4044, 7403635 Ness Ziona (IL). BEIMAN, Merav PCT/US2018/053583 [EilL]; c/o ImmPACT-Bio Ltd., 2 Ilian Ramon St., P.O. (22) International Filing Date: Box 4044, 7403635 Ness Ziona (E.). 28 September 2018 (28.09.2018) (74) Agent: MACDOUGALL, Christina, A. et al; Morgan, (25) Filing Language: English Lewis & Bockius LLP, One Market, Spear Tower, SanFran- cisco, CA 94105 (US). (26) Publication Language: English (81) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of national protection available): AE, AG, AL, AM, 62/564,454 28 September 2017 (28.09.2017) US AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, 62/649,429 28 March 2018 (28.03.2018) US CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, (71) Applicant: IMMP ACT-BIO LTD. -

Sean Raspet – Molecules
1. Commercial name: Fructaplex© IUPAC Name: 2-(3,3-dimethylcyclohexyl)-2,5,5-trimethyl-1,3-dioxane SMILES: CC1(C)CCCC(C1)C2(C)OCC(C)(C)CO2 Molecular weight: 240.39 g/mol Volume (cubic Angstroems): 258.88 Atoms number (non-hydrogen): 17 miLogP: 4.43 Structure: Biological Properties: Predicted Druglikenessi: GPCR ligand -0.23 Ion channel modulator -0.03 Kinase inhibitor -0.6 Nuclear receptor ligand 0.15 Protease inhibitor -0.28 Enzyme inhibitor 0.15 Commercial name: Fructaplex© IUPAC Name: 2-(3,3-dimethylcyclohexyl)-2,5,5-trimethyl-1,3-dioxane SMILES: CC1(C)CCCC(C1)C2(C)OCC(C)(C)CO2 Predicted Olfactory Receptor Activityii: OR2L13 83.715% OR1G1 82.761% OR10J5 80.569% OR2W1 78.180% OR7A2 77.696% 2. Commercial name: Sylvoxime© IUPAC Name: N-[4-(1-ethoxyethenyl)-3,3,5,5tetramethylcyclohexylidene]hydroxylamine SMILES: CCOC(=C)C1C(C)(C)CC(CC1(C)C)=NO Molecular weight: 239.36 Volume (cubic Angstroems): 252.83 Atoms number (non-hydrogen): 17 miLogP: 4.33 Structure: Biological Properties: Predicted Druglikeness: GPCR ligand -0.6 Ion channel modulator -0.41 Kinase inhibitor -0.93 Nuclear receptor ligand -0.17 Protease inhibitor -0.39 Enzyme inhibitor 0.01 Commercial name: Sylvoxime© IUPAC Name: N-[4-(1-ethoxyethenyl)-3,3,5,5tetramethylcyclohexylidene]hydroxylamine SMILES: CCOC(=C)C1C(C)(C)CC(CC1(C)C)=NO Predicted Olfactory Receptor Activity: OR52D1 71.900% OR1G1 70.394% 0R52I2 70.392% OR52I1 70.390% OR2Y1 70.378% 3. Commercial name: Hyperflor© IUPAC Name: 2-benzyl-1,3-dioxan-5-one SMILES: O=C1COC(CC2=CC=CC=C2)OC1 Molecular weight: 192.21 g/mol Volume -

Preview of “Supplement Table S4.Xls”
Annotations for all recurrently lost regions cytoband 1p36.12 3p14.2 3q13.13 q value 0,01352 0,1513 0,085307 residual q value 0,01352 0,1513 0,085307 wide peak boundaries chr1:23457835-23714048 chr3:60396160-60637030 chr3:110456383-110657226 genes in wide peak E2F2 FHIT DPPA4 ID3 DPPA2 TCEA3 DDEFL1 TBC1D19 PI4K2B STIM2 GBA3 KCNIP4 DKFZp761B107 C4orf28 GPR125 FLJ45721 hsa-mir-218-1 CXCL3 GRSF1 HNRPD HTN1 HTN3 IBSP IGFBP7 IGJ IL8 CXCL10 KDR CXCL9 AFF1 MUC7 NKX6-1 PF4 PF4V1 PKD2 POLR2B PPEF2 PPAT PPBP PRKG2 MAPK10 PTPN13 REST CXCL6 CXCL11 CXCL5 SPINK2 SPP1 SRP72 STATH SULT1E1 UGT2B4 UGT2B7 UGT2B10 UGT2B15 UGT2B17 SPARCL1 VDP SLC4A4 HERC3 GENX-3414 CDKL2 TMPRSS11D ABCG2 ADAMTS3 CLOCK CEP135 G3BP2 HNRPDL ENAM FAM13A1 CXCL13 PAICS UGT2B11 HPSE NMU SMR3B NPFFR2 UGT2A1 CCNI hsa-mir-491 hsa-mir-31 4p15.32 4q13.1 4q13.1 (continued) 6q14.1 0,14888 0,17878 0,093693 0,14888 0,17878 0,093693 chr4:17969802-29966659 chr4:55756035-90966136 chr6:76830186-107898353 CCKAR AFM SEC31A AIM1 DHX15 AFP RUFY3 BCKDHB RBPSUH ALB WDFY3 PRDM1 SOD3 AMBN LPHN3 CCNC SLIT2 ANXA3 DKFZP564O0823 CGA SLC34A2 AREG RCHY1 CNR1 PPARGC1A ART3 ANKRD17 EPHA7 KIAA0746 BMP3 BRDG1 GABRR1 ANAPC4 BTC SMR3A GABRR2 SLA/LP CCNG2 ASAHL GRIK2 LGI2 SCARB2 COQ2 HTR1B TBC1D19 CDS1 SULT1B1 HTR1E PI4K2B CENPC1 TMPRSS11E IMPG1 STIM2 CSN1S1 MRPS18C ME1 GBA3 CSN2 COPS4 NT5E KCNIP4 CSN3 HSD17B11 PGM3 DKFZp761B107 DCK HERC5 POU3F2 C4orf28 DMP1 PLAC8 PREP GPR125 DSPP NUDT9 SIM1 FLJ45721 EPHA5 NUP54 ELOVL4 hsa-mir-218-1 EREG UGT2B28 MAP3K7 FGF5 ODAM TPBG GC HERC6 TTK GK2 SDAD1 RNGTT GNRHR UBE1L2 TBX18 -

A Comprehensive Analysis of Differentially Expressed Genes and Pathways in Abdominal Aortic Aneurysm
MOLECULAR MEDICINE REPORTS 12: 2707-2714, 2015 A comprehensive analysis of differentially expressed genes and pathways in abdominal aortic aneurysm KAI YUAN, WEI LIANG and JIWEI ZHANG Department of Vascular Surgery, Renji Hospital of Shanghai Jiao Tong University School of Medicine, Shanghai 200127, P.R. China Received May 29, 2014; Accepted March 3, 2015 DOI: 10.3892/mmr.2015.3709 Abstract. The current study aimed to investigate the molecular inal aorta exceeding the normal aortic diameter by >50% (1). mechanism underlying abdominal aortic aneurysm (AAA) The most severe complication of AAA is AAA rupture, via various bioinformatics techniques. Gene expression which is the tenth primary cause of mortality for American profiling analysis of differentially expressed genes (DEGs) Caucasian males between 65-74 years old (2). As AAA rupture between AAA samples and normal controls was conducted. is fatal within minutes, in the majority of cases, the patients do The Database for Annotation, Visualization and Integrated not reach hospital in time to receive treatment. Therefore, the Discovery tool was utilized to perform Gene ontology (GO) mortality rate of AAA rupture is ~90% (3). and Kyoto Encyclopedia of Genes and Genomes analyses for Thus, there is an urgent requirement for the development DEGs and clusters from the protein-protein interaction (PPI) of effective therapies for AAA, and elucidating the etiology network, which was constructed using the Search Tool for the of AAA is important in this development. Chronic tobacco Retrieval of Interacting Genes. In addition, important tran- smoking has been identified as the most important risk factor scription factors (TFs) that regulated DEGs were investigated. -

The Hypothalamus As a Hub for SARS-Cov-2 Brain Infection and Pathogenesis
bioRxiv preprint doi: https://doi.org/10.1101/2020.06.08.139329; this version posted June 19, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. The hypothalamus as a hub for SARS-CoV-2 brain infection and pathogenesis Sreekala Nampoothiri1,2#, Florent Sauve1,2#, Gaëtan Ternier1,2ƒ, Daniela Fernandois1,2 ƒ, Caio Coelho1,2, Monica ImBernon1,2, Eleonora Deligia1,2, Romain PerBet1, Vincent Florent1,2,3, Marc Baroncini1,2, Florence Pasquier1,4, François Trottein5, Claude-Alain Maurage1,2, Virginie Mattot1,2‡, Paolo GiacoBini1,2‡, S. Rasika1,2‡*, Vincent Prevot1,2‡* 1 Univ. Lille, Inserm, CHU Lille, Lille Neuroscience & Cognition, DistAlz, UMR-S 1172, Lille, France 2 LaBoratorY of Development and PlasticitY of the Neuroendocrine Brain, FHU 1000 daYs for health, EGID, School of Medicine, Lille, France 3 Nutrition, Arras General Hospital, Arras, France 4 Centre mémoire ressources et recherche, CHU Lille, LiCEND, Lille, France 5 Univ. Lille, CNRS, INSERM, CHU Lille, Institut Pasteur de Lille, U1019 - UMR 8204 - CIIL - Center for Infection and ImmunitY of Lille (CIIL), Lille, France. # and ƒ These authors contriButed equallY to this work. ‡ These authors directed this work *Correspondence to: [email protected] and [email protected] Short title: Covid-19: the hypothalamic hypothesis 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.06.08.139329; this version posted June 19, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Us 2018 / 0305689 A1
US 20180305689A1 ( 19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2018 /0305689 A1 Sætrom et al. ( 43 ) Pub . Date: Oct. 25 , 2018 ( 54 ) SARNA COMPOSITIONS AND METHODS OF plication No . 62 /150 , 895 , filed on Apr. 22 , 2015 , USE provisional application No . 62/ 150 ,904 , filed on Apr. 22 , 2015 , provisional application No. 62 / 150 , 908 , (71 ) Applicant: MINA THERAPEUTICS LIMITED , filed on Apr. 22 , 2015 , provisional application No. LONDON (GB ) 62 / 150 , 900 , filed on Apr. 22 , 2015 . (72 ) Inventors : Pål Sætrom , Trondheim (NO ) ; Endre Publication Classification Bakken Stovner , Trondheim (NO ) (51 ) Int . CI. C12N 15 / 113 (2006 .01 ) (21 ) Appl. No. : 15 /568 , 046 (52 ) U . S . CI. (22 ) PCT Filed : Apr. 21 , 2016 CPC .. .. .. C12N 15 / 113 ( 2013 .01 ) ; C12N 2310 / 34 ( 2013. 01 ) ; C12N 2310 /14 (2013 . 01 ) ; C12N ( 86 ) PCT No .: PCT/ GB2016 /051116 2310 / 11 (2013 .01 ) $ 371 ( c ) ( 1 ) , ( 2 ) Date : Oct . 20 , 2017 (57 ) ABSTRACT The invention relates to oligonucleotides , e . g . , saRNAS Related U . S . Application Data useful in upregulating the expression of a target gene and (60 ) Provisional application No . 62 / 150 ,892 , filed on Apr. therapeutic compositions comprising such oligonucleotides . 22 , 2015 , provisional application No . 62 / 150 ,893 , Methods of using the oligonucleotides and the therapeutic filed on Apr. 22 , 2015 , provisional application No . compositions are also provided . 62 / 150 ,897 , filed on Apr. 22 , 2015 , provisional ap Specification includes a Sequence Listing . SARNA sense strand (Fessenger 3 ' SARNA antisense strand (Guide ) Mathew, Si Target antisense RNA transcript, e . g . NAT Target Coding strand Gene Transcription start site ( T55 ) TY{ { ? ? Targeted Target transcript , e . -
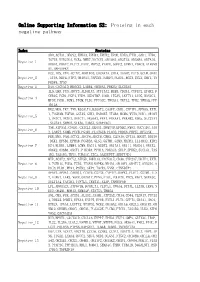
Online Supporting Information S2: Proteins in Each Negative Pathway
Online Supporting Information S2: Proteins in each negative pathway Index Proteins ADO,ACTA1,DEGS2,EPHA3,EPHB4,EPHX2,EPOR,EREG,FTH1,GAD1,HTR6, IGF1R,KIR2DL4,NCR3,NME7,NOTCH1,OR10S1,OR2T33,OR56B4,OR7A10, Negative_1 OR8G1,PDGFC,PLCZ1,PROC,PRPS2,PTAFR,SGPP2,STMN1,VDAC3,ATP6V0 A1,MAPKAPK2 DCC,IDS,VTN,ACTN2,AKR1B10,CACNA1A,CHIA,DAAM2,FUT5,GCLM,GNAZ Negative_2 ,ITPA,NEU4,NTF3,OR10A3,PAPSS1,PARD3,PLOD1,RGS3,SCLY,SHC1,TN FRSF4,TP53 Negative_3 DAO,CACNA1D,HMGCS2,LAMB4,OR56A3,PRKCQ,SLC25A5 IL5,LHB,PGD,ADCY3,ALDH1A3,ATP13A2,BUB3,CD244,CYFIP2,EPHX2,F CER1G,FGD1,FGF4,FZD9,HSD17B7,IL6R,ITGAV,LEFTY1,LIPG,MAN1C1, Negative_4 MPDZ,PGM1,PGM3,PIGM,PLD1,PPP3CC,TBXAS1,TKTL2,TPH2,YWHAQ,PPP 1R12A HK2,MOS,TKT,TNN,B3GALT4,B3GAT3,CASP7,CDH1,CYFIP1,EFNA5,EXTL 1,FCGR3B,FGF20,GSTA5,GUK1,HSD3B7,ITGB4,MCM6,MYH3,NOD1,OR10H Negative_5 1,OR1C1,OR1E1,OR4C11,OR56A3,PPA1,PRKAA1,PRKAB2,RDH5,SLC27A1 ,SLC2A4,SMPD2,STK36,THBS1,SERPINC1 TNR,ATP5A1,CNGB1,CX3CL1,DEGS1,DNMT3B,EFNB2,FMO2,GUCY1B3,JAG Negative_6 2,LARS2,NUMB,PCCB,PGAM1,PLA2G1B,PLOD2,PRDX6,PRPS1,RFXANK FER,MVD,PAH,ACTC1,ADCY4,ADCY8,CBR3,CLDN16,CPT1A,DDOST,DDX56 ,DKK1,EFNB1,EPHA8,FCGR3A,GLS2,GSTM1,GZMB,HADHA,IL13RA2,KIR2 Negative_7 DS4,KLRK1,LAMB4,LGMN,MAGI1,NUDT2,OR13A1,OR1I1,OR4D11,OR4X2, OR6K2,OR8B4,OXCT1,PIK3R4,PPM1A,PRKAG3,SELP,SPHK2,SUCLG1,TAS 1R2,TAS1R3,THY1,TUBA1C,ZIC2,AASDHPPT,SERPIND1 MTR,ACAT2,ADCY2,ATP5D,BMPR1A,CACNA1E,CD38,CYP2A7,DDIT4,EXTL Negative_8 1,FCER1G,FGD3,FZD5,ITGAM,MAPK8,NR4A1,OR10V1,OR4F17,OR52D1,O R8J3,PLD1,PPA1,PSEN2,SKP1,TACR3,VNN1,CTNNBIP1 APAF1,APOA1,CARD11,CCDC6,CSF3R,CYP4F2,DAPK1,FLOT1,GSTM1,IL2 -

Supplementary Methods
doi: 10.1038/nature06162 SUPPLEMENTARY INFORMATION Supplementary Methods Cloning of human odorant receptors 423 human odorant receptors were cloned with sequence information from The Olfactory Receptor Database (http://senselab.med.yale.edu/senselab/ORDB/default.asp). Of these, 335 were predicted to encode functional receptors, 45 were predicted to encode pseudogenes, 29 were putative variant pairs of the same genes, and 14 were duplicates. We adopted the nomenclature proposed by Doron Lancet 1. OR7D4 and the six intact odorant receptor genes in the OR7D4 gene cluster (OR1M1, OR7G2, OR7G1, OR7G3, OR7D2, and OR7E24) were used for functional analyses. SNPs in these odorant receptors were identified from the NCBI dbSNP database (http://www.ncbi.nlm.nih.gov/projects/SNP) or through genotyping. OR7D4 single nucleotide variants were generated by cloning the reference sequence from a subject or by inducing polymorphic SNPs by site-directed mutagenesis using overlap extension PCR. Single nucleotide and frameshift variants for the six intact odorant receptors in the same gene cluster as OR7D4 were generated by cloning the respective genes from the genomic DNA of each subject. The chimpanzee OR7D4 orthologue was amplified from chimpanzee genomic DNA (Coriell Cell Repositories). Odorant receptors that contain the first 20 amino acids of human rhodopsin tag 2 in pCI (Promega) were expressed in the Hana3A cell line along with a short form of mRTP1 called RTP1S, (M37 to the C-terminal end), which enhances functional expression of the odorant receptors 3. For experiments with untagged odorant receptors, OR7D4 RT and S84N variants without the Rho tag were cloned into pCI. -

Research/0018.1
http://genomebiology.com/2001/2/6/research/0018.1 Research The human olfactory receptor repertoire comment Sergey Zozulya, ernando Echeverri and Trieu Nguyen Address: Senomyx Inc., 11099 North Torrey Pines Road, La Jolla, CA 92037, USA. Correspondence: Sergey Zozulya. E-mail: [email protected] reviews Published: 1 June 2001 Received: 8 March 2001 Revised: 12 April 2001 Genome Biology 2001, 2(6):research0018.1–0018.12 Accepted: 18 April 2001 The electronic version of this article is the complete one and can be found online at http://genomebiology.com/2001/2/6/research/0018 © 2001 Zozulya et al., licensee BioMed Central Ltd (Print ISSN 1465-6906; Online ISSN 1465-6914) reports Abstract Background: The mammalian olfactory apparatus is able to recognize and distinguish thousands of structurally diverse volatile chemicals. This chemosensory function is mediated by a very large family of seven-transmembrane olfactory (odorant) receptors encoded by approximately 1,000 genes, the majority of which are believed to be pseudogenes in humans. deposited research Results: The strategy of our sequence database mining for full-length, functional candidate odorant receptor genes was based on the high overall sequence similarity and presence of a number of conserved sequence motifs in all known mammalian odorant receptors as well as the absence of introns in their coding sequences. We report here the identification and physical cloning of 347 putative human full-length odorant receptor genes. Comparative sequence analysis of the predicted gene products allowed us to identify and define a number of consensus sequence motifs and structural features of this vast family of receptors. -

Association of Somatic Mutations of ADAMTS Genes with Improved Chemotherapy Sensitivity and Survival in High-Grade Serous Ovarian Carcinoma
Supplementary Online Content Liu Y, Yasukawa M, Chen K, et al. Association of somatic mutations of ADAMTS genes with improved chemotherapy sensitivity and survival in high-grade serous ovarian carcinoma. JAMA Oncol. Published online June 11, 2015. doi:10.1001/jamaoncol.2015.1432 eMethods eFigure 1. Analysis flow chart for identification and validation of ADAMTS mutations eFigure 2. Responder-related genes identified in the TCGA discovery cohort eFigure 3. Distribution of protein alterations encoded in the ADAMTS genes in the TCGA discovery cohort eFigure 4. Association of BRCA mutations with survival and chemotherapy response in the TCGA discovery cohort eFigure 5. Association of residual tumor size with survival and chemotherapy response in the TCGA discovery cohort. eFigure 6. Association of ADAMTS mutations with genetic instability in the TCGA discovery cohort. eFigure 7. Comparison of association of ADAMTS and BRCA1/2 mutations with mutation frequency in the TCGA discovery cohort eFigure 8. Association of ADAMTS mutations with mutation spectra in the TCGA discovery cohort eFigure 9. Correlation of mutation frequency with C>T or A>T fractions in the TCGA discovery cohort eFigure 10. Distribution of the number of patients harboring gene mutations among the 105 random gene selections eFigure 11. Statistical significance (P value) of outcome association among the 105 random gene selections eFigure 12. Distribution of OvCa patients from different tissue source sites in the TCGA discovery cohort and validation cohort eFigure 13. ADAMTS and BRCA1/2 mutations in the TCGA validation cohort © 2015 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 09/27/2021 eFigure 14.