Aneuploidy Increases Resistance to Chemotherapeutics by Antagonizing Cell Division
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chromosome 18
Chromosome 18 Description Humans normally have 46 chromosomes in each cell, divided into 23 pairs. Two copies of chromosome 18, one copy inherited from each parent, form one of the pairs. Chromosome 18 spans about 78 million DNA building blocks (base pairs) and represents approximately 2.5 percent of the total DNA in cells. Identifying genes on each chromosome is an active area of genetic research. Because researchers use different approaches to predict the number of genes on each chromosome, the estimated number of genes varies. Chromosome 18 likely contains 200 to 300 genes that provide instructions for making proteins. These proteins perform a variety of different roles in the body. Health Conditions Related to Chromosomal Changes The following chromosomal conditions are associated with changes in the structure or number of copies of chromosome 18. Distal 18q deletion syndrome Distal 18q deletion syndrome occurs when a piece of the long (q) arm of chromosome 18 is missing. The term "distal" means that the missing piece (deletion) occurs near one end of the chromosome arm. The signs and symptoms of distal 18q deletion syndrome include delayed development and learning disabilities, short stature, weak muscle tone ( hypotonia), foot abnormalities, and a wide variety of other features. The deletion that causes distal 18q deletion syndrome can occur anywhere between a region called 18q21 and the end of the chromosome. The size of the deletion varies among affected individuals. The signs and symptoms of distal 18q deletion syndrome are thought to be related to the loss of multiple genes from this part of the long arm of chromosome 18. -

First Case Report of Maternal Mosaic Tetrasomy 9P Incidentally Detected on Non-Invasive Prenatal Testing
G C A T T A C G G C A T genes Article First Case Report of Maternal Mosaic Tetrasomy 9p Incidentally Detected on Non-Invasive Prenatal Testing Wendy Shu 1,*, Shirley S. W. Cheng 2 , Shuwen Xue 3, Lin Wai Chan 1, Sung Inda Soong 4, Anita Sik Yau Kan 5 , Sunny Wai Hung Cheung 6 and Kwong Wai Choy 3,* 1 Department of Obstetrics and Gynaecology, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong, China; [email protected] 2 Clinical Genetic Service, Hong Hong Children Hospital, Ngau Tau Kok, Hong Kong, China; [email protected] 3 Department of Obstetrics and Gynaecology, Chinese University of Hong Kong, Hong Kong, China; [email protected] 4 Department of Clinical Oncology, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong, China; [email protected] 5 Prenatal Diagnostic Laboratory, Tsan Yuk Hospital, Sai Ying Pun, Hong Kong, China; [email protected] 6 NIPT Department, NGS Lab, Xcelom Limited, Hong Kong, China; [email protected] * Correspondence: [email protected] (W.S.); [email protected] (K.W.C.); Tel.: +852-25-957-359 (W.S.); +852-35-053-099 (K.W.C.) Abstract: Tetrasomy 9p (ORPHA:3390) is a rare syndrome, hallmarked by growth retardation; psychomotor delay; mild to moderate intellectual disability; and a spectrum of skeletal, cardiac, renal and urogenital defects. Here we present a Chinese female with good past health who conceived her pregnancy naturally. Non-invasive prenatal testing (NIPT) showed multiple chromosomal aberrations were consistently detected in two sampling times, which included elevation in DNA from Citation: Shu, W.; Cheng, S.S.W.; chromosome 9p. -

Aneuploidy and Aneusomy of Chromosome 7 Detected by Fluorescence in Situ Hybridization Are Markers of Poor Prognosis in Prostate Cancer'
[CANCERRESEARCH54,3998-4002,August1, 19941 Advances in Brief Aneuploidy and Aneusomy of Chromosome 7 Detected by Fluorescence in Situ Hybridization Are Markers of Poor Prognosis in Prostate Cancer' Antonio Alcaraz, Satoru Takahashi, James A. Brown, John F. Herath, Erik J- Bergstralh, Jeffrey J. Larson-Keller, Michael M Lieber, and Robert B. Jenkins2 Depart,nent of Urology [A. A., S. T., J. A. B., M. M. U, Laboratory Medicine and Pathology (J. F. H., R. B. fl, and Section of Biostatistics (E. J. B., J. J. L-JCJ, Mayo Clinic and Foundation@ Rochester, Minnesota 55905 Abstract studies on prostate carcinoma samples. Interphase cytogenetic analy sis using FISH to enumerate chromosomes has the potential to over Fluorescence in situ hybridization is a new methodologj@which can be come many of the difficulties associated with traditional cytogenetic used to detect cytogenetic anomalies within interphase tumor cells. We studies. Previous studies from this institution have demonstrated that used this technique to identify nonrandom numeric chromosomal alter ations in tumor specimens from the poorest prognosis patients with path FISH analysis with chromosome enumeration probes is more sensitive ological stages T2N@M,Jand T3NOMOprostate carcinomas. Among 1368 than FCM for detecting aneuploid prostate cancers (4, 5, 7). patients treated by radical prostatectomy, 25 study patients were ascer We designed a case-control study to test the hypothesis that spe tamed who died most quickly from progressive prostate carcinoma within cific, nonrandom cytogenetic changes are present in tumors removed 3 years of diagnosis and surgery. Tumors from 25 control patients who from patients with prostate carcinomas with poorest prognoses . -

General Contribution
24 Abstracts of 37th Annual Meeting A1 A SCREENING METHOD FOR FRAGILE X MUTATION: DETECTION OF THE CGG REPEAT IN FMR-1 GENE BY PCR WITH BIOTIN-LABELED PRIMER. ..Eiji NANBA, Kousaku OHNO and Kenzo TAKESHITA Division of Child Neurology, Institute of Neurological Sciences, Tot- tori University School of Medicine. Yonago We have developed a new polymerase chain reaction(PCR)-based method for detection of the CGG repeat in FMR-1 gene. No specific product from PCR was detected on the gel with ethidium bromide staining, because 7-deaza-2'-dGTP is necessary for amplification of this repeat. Biotin-labeled primer was used for PCR and the product was transferred to a nylon membrane followed the detection of biotin by Smilight kit. The size of PCR product from normal control were slightly various and around 300bp. No PCR product was detected from 3 fragile X male patients in 2 families diagnosed by cytogenetic examination. This method is useful for genetic screen- ing of male mental retardation patients to exclude the fragile X mutation. A2 DNA ANALYSISFOR FRAGILE X SYNDROME Osamu KOSUDA,Utak00GASA, ~.ideynki INH, a~ji K/NAGIJCltI, and Kazumasa ]tIKIJI (SILL Inc., Tokyo) Fragile X syndrome is X-linked disease having the amplification of (CG6)n repeat sequence in the chromsomeXq27.3. We performed Southern blot analysis using three probes recognized repetitive sequence resion. Normal controle showed 5.2Kb with Eco RI digest and 2.7Kb with Eco RI/Bss ttII digest as the germ tines by the Southern blot analysis. However, three cell lines established fro~ unrelated the patients with fragile X showed the abnormal bands between 5.2 and 7.7Kb with Eco RI digest, and between 2.7 and 7.7Kb with Eco aI/Bss HII digest. -

Tetrasomy 9P Syndrome in a Filipino Infant
CASE REPORT Tetrasomy 9p Syndrome in a Filipino Infant Ebner Bon G. Maceda,1,2 Erena S. Kasahara,3 Edsel Allan G. Salonga,2 Myrian R. Dela Cruz2 and Leniza De Castro-Hamoy1,2 1Division of Clinical Genetics, Department of Pediatrics, College of Medicine and Philippine General Hospital, University of the Philippines Manila 2Institute of Human Genetics, National Institutes of Health, University of the Philippines Manila 3Division of Newborn Medicine, Department of Pediatrics, College of Medicine and Philippine General Hospital, University of the Philippines Manila ABSTRACT Tetrasomy 9p syndrome is a rare chromosomal abnormality syndrome whose most common features include hypertelorism, malformed ears, bulbous nose and microretrognathia. These features present as a result of an additional two copies of the short arm of chromosome 9. Here we present a neonate with characteristic facial features of hypertelorism, downslanted palpebral fissure, bulbous nose, small cupped ears, cleft lip and palate, and downturned corners of the mouth. Clinical features were consistent with the cytogenetic analysis of tetrasomy 9p. In general, clinicians are not as familiar with the features of tetrasomy 9p syndrome as that of more common chromosomal abnormalities like trisomies 13, 18, and 21. Hence, this case re-emphasizes the importance of doing the standard karyotyping for patients presenting with multiple congenital anomalies. Also, this is the first reported case of Tetrasomy 9p syndrome in Filipinos. Key Words: Tetrasomy 9p, isochromosome, hypertelorism, bulbous nose INTRODUCTION Tetrasomy 9p is a broad term used to describe the presence of a supernumerary chromosome of the short arm of chromosome 9. In more specific terms, the presence of 2 extra copies of 9p arms can be either isodicentric or pseudodicentric. -

Cytogenetics, Chromosomal Genetics
Cytogenetics Chromosomal Genetics Sophie Dahoun Service de Génétique Médicale, HUG Geneva, Switzerland [email protected] Training Course in Sexual and Reproductive Health Research Geneva 2010 Cytogenetics is the branch of genetics that correlates the structure, number, and behaviour of chromosomes with heredity and diseases Conventional cytogenetics Molecular cytogenetics Molecular Biology I. Karyotype Definition Chromosomal Banding Resolution limits Nomenclature The metaphasic chromosome telomeres p arm q arm G-banded Human Karyotype Tjio & Levan 1956 Karyotype: The characterization of the chromosomal complement of an individual's cell, including number, form, and size of the chromosomes. A photomicrograph of chromosomes arranged according to a standard classification. A chromosome banding pattern is comprised of alternating light and dark stripes, or bands, that appear along its length after being stained with a dye. A unique banding pattern is used to identify each chromosome Chromosome banding techniques and staining Giemsa has become the most commonly used stain in cytogenetic analysis. Most G-banding techniques require pretreating the chromosomes with a proteolytic enzyme such as trypsin. G- banding preferentially stains the regions of DNA that are rich in adenine and thymine. R-banding involves pretreating cells with a hot salt solution that denatures DNA that is rich in adenine and thymine. The chromosomes are then stained with Giemsa. C-banding stains areas of heterochromatin, which are tightly packed and contain -

Congenital Heart Disease and Chromossomopathies Detected By
Review Article DOI: 10.1590/0103-0582201432213213 Congenital heart disease and chromossomopathies detected by the karyotype Cardiopatias congênitas e cromossomopatias detectadas por meio do cariótipo Cardiopatías congénitas y anomalías cromosómicas detectadas mediante cariotipo Patrícia Trevisan1, Rafael Fabiano M. Rosa2, Dayane Bohn Koshiyama1, Tatiana Diehl Zen1, Giorgio Adriano Paskulin1, Paulo Ricardo G. Zen1 ABSTRACT Conclusions: Despite all the progress made in recent de- cades in the field of cytogenetic, the karyotype remains an es- Objective: To review the relationship between congenital sential tool in order to evaluate patients with congenital heart heart defects and chromosomal abnormalities detected by disease. The detailed dysmorphological physical examination the karyotype. is of great importance to indicate the need of a karyotype. Data sources: Scientific articles were searched in MED- LINE database, using the descriptors “karyotype” OR Key-words: heart defects, congenital; karyotype; Down “chromosomal” OR “chromosome” AND “heart defects, syndrome; trisomy; chromosome aberrations. congenital”. The research was limited to articles published in English from 1980 on. RESUMO Data synthesis: Congenital heart disease is characterized by an etiologically heterogeneous and not well understood Objetivo: Realizar uma revisão da literatura sobre a group of lesions. Several researchers have evaluated the pres- relação das cardiopatias congênitas com anormalidades ence of chromosomal abnormalities detected by the karyo- cromossômicas detectadas por meio do exame de cariótipo. type in patients with congenital heart disease. However, Fontes de dados: Pesquisaram-se artigos científicos no most of the articles were retrospective studies developed in portal MEDLINE, utilizando-se os descritores “karyotype” Europe and only some of the studied patients had a karyo- OR “chromosomal” OR “chromosome” AND “heart defects, type exam. -
Possible Misdiagnosis of 46,XX Testicular Disorders of Sex
Int. J. Med. Sci. 2020, Vol. 17 1136 Ivyspring International Publisher International Journal of Medical Sciences 2020; 17(9): 1136-1141. doi: 10.7150/ijms.46058 Research Paper Possible misdiagnosis of 46,XX testicular disorders of sex development in infertile males Tong Chen1,2†, Linlin Tian1,3†, Xianlong Wang1, Demin Fan4, Gang Ma1, Rong Tang1, Xujun Xuan1,5 1. Center for Reproductive Medicine, Shandong University, National Research Center for Assisted Reproductive Technology and Reproductive Genetics; The Key Laboratory for Reproductive Endocrinology of Ministry of Education, Jinan, Shandong 250021, P.R. China 2. Department of Pediatric Surgery, Shanghai Children's Hospital, Shanghai Jiao Tong University, Shanghai, 200062, P.R. China 3. Department of microbiology, Faculty of Basic Medical Sciences, Guilin Medical University, Guilin, Guangxi 541004, P.R. China 4. Department of Urology, Shandong Provincial Qianfoshan Hospital, Jinan, Shandong 250002, P.R. China 5. Department of Andrology, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, 518107, P.R. China. †Tong Chen and Linlin Tian contributed equally to this study. Corresponding author: Xujun Xuan, Department of Andrology, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, 518107, P.R. China. E-mail: [email protected] © The author(s). This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See http://ivyspring.com/terms for full terms and conditions. Received: 2020.03.16; Accepted: 2020.04.23; Published: 2020.05.11 Abstract Objectives: The 46,XX disorders of sex development (DSD) is a rare genetic cause of male infertility and possible misdiagnosis of this condition has never been reported. -

Autism Spectrum Disorder Associated with 49,XYYYY: Case Report and Review of the Literature
Demily et al. BMC Medical Genetics (2017) 18:9 DOI 10.1186/s12881-017-0371-1 CASEREPORT Open Access Autism spectrum disorder associated with 49,XYYYY: case report and review of the literature Caroline Demily1*, Alice Poisson1, Elodie Peyroux1, Valérie Gatellier1, Alain Nicolas2, Caroline Rigard1, Caroline Schluth-Bolard3, Damien Sanlaville3 and Massimiliano Rossi3 Abstract Background: Sex chromosome aneuploidies occur in approximately one in 420 live births. The most frequent abnormalities are 45,X (Turner syndrome), 47,XXX (triple X), 47,XXY (Klinefelter syndrome), and 47,XYY. The prevalence of males with more than one extra sex chromosome (e.g. 48,XXYY or 48,XXXY) is less common. However, the literature provides little information about the cognitive and behavioural phenotype and the natural history of the disease. We report the clinical, neurocognitive, social cognitive and psychiatric characterization of a patient with 49,XYYYY syndrome. Case presentation: The patient presented with a complex phenotype including a particular cognitive profile with intellectual deficiency and autism spectrum disorder (ASD) with limited interests. Moreover, social anxiety disorder with selective mutism and separation anxiety disorder were observed (DSM-5 criteria, MINI Assessment). Conclusion: It is now admitted that 49,XYYYY has unique medical, neurodevelopmental and behavioural characteristics. Interestingly, ASD is more common in groups with Y chromosome aneuploidy. This clinical report suggests that understanding the cognitive and social functioning of these patients may provide new insights into possible therapeutic strategies, as cognitive remediation or social cognitive training. Keywords: XYYYY, Autism spectrum disorder, Social cognition, Neurocognition, Behavioural disorders Background 48,XYYY and 49,XYYYY karyotypes are very rare: less Sex chromosome aneuploidies (SCA) occur in approxi- than ten cases have been described for 48,XYYY and mately one in 420 live births [1]. -

Cell Biology
Course Code-EDU161 and Course Name:Botany Aneuploidy: Meaning, Forms and Importance | Cell Biology Meaning of Aneuploidy: Aneuploidy is the presence of chromosome number that is different from the simple multiple of the basic chromosome number. An organism which contains one or more incomplete chromosome sets is known as aneuploid. Aneuploidy can be either due to loss of one or more chromosomes (hypo-ploidy) or due to addition of one or more chromosomes to complete chromosome complement (hyper-ploidy). Hypo-ploidy may be due to loss of a single chromosome – monosomy (2n – 1), or due to loss of one pair of chromosomes – nullisomy (2n – 2). Similarly, hyper-ploidy may involve addition of either a single chromosome-trisomy (2n + 1) or a pair of chromosomes (2n + 2) – tetrasomy (Fig. 11.2). Forms of Aneuploidy: Monosomy: Monosomy is the phenomenon where an individual lacks one or a few non-homologous chromosome(s) of a diploid complement Types of Monosomy: Single monosomies lack one complete chromosome (2n – 1), these create major imbalance and may not be tolerated in diploids. Monosomies, however, are viable in polyploid species, in which the loss of one chromosome has a less marked effect. For instance, in ordinary tobacco, Nicotiana tabacum, which is a tetraploid species with 2n = 48 chromosomes, a series of different monosomic types with 47 chromosomes is known. The number of possible monosomies in an organism is equal to the haploid chromosome number. Double monosomies (2n – 1 – 1) or triple monosomies (2n – 1 – 1 – 1) could also be produced in polyploids. Origin of Monosomy: The origin of the monosomies may be from the production of n – 1 types of gametes due to rare nondisjunction of a bivalent. -
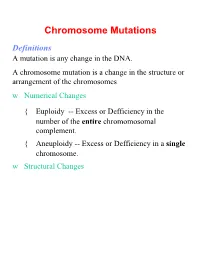
Chromosome Mutations Definitions a Mutation Is Any Change in the DNA
Chromosome Mutations Definitions A mutation is any change in the DNA. A chromosome mutation is a change in the structure or arrangement of the chromosomes w Numerical Changes { Euploidy -- Excess or Defficiency in the number of the entire chromomosomal complement. { Aneuploidy -- Excess or Defficiency in a single chromosome. w Structural Changes Numerical Chromosome Mutations Euploid -- “True Number” The cell(s) have the same number of copies of all the chromosome. 1. Monoploid (Haploid if a gamete) 2. Diploid (Often is not considered a Euploid) 3. Triploid 4. Tetraploid Euploidy Chromosomes shown at metaphase Normal Diploid Euploid Monoploid Triploid Tetraploid Euploidy is an excess or deficiency for the entire complement of chromosomes. Euploidy Organisms w Plants, amphibians, lower reptiles w Some cells in other organisms (liver, midgut, etc.) Root Causes w Nondisjunction w Autoreduplication Euploidy (continued) Origins w Autopolyploidy { Often involving individuals within the same species. { Infertile. Propagation by cloning. w Allopolyploidy { Often involving individuals from different species. { Fertile. Dosage Concerns Examples Numerical Chromosome Mutations Aneuploidy Excess or Defficiency in a single chromosome. 1. Monosomy 2. Disomy (only if normally cell is not diploid) 3. Trisomy 4. Tetrasomy Aneuploidy Chromosomes shown at metaphase Normal Diploid Aneuploid Monosomic Trisomic Tetrasomic Aneuploidy is an excess or deficiency for fewer than the entire complement of chromosomes. Aneuploidy Organisms w All organisms w Variability in how they deal with it Root Causes w Nondisjunction w Results of Chromosome Structure Mutations Aneuploidy -- Examples Plants w Jimson Weed (Datura stramonium) Animals w Drosophila: Triploid females are viable & fertile. Trisomy flies are not! w Human (autosomal): { Trisomy 13 (Patau Syndrome) 1:20,000 { Trisomy 18 (Edwards Syndrome) 1:10,000 { Trisomy 21 (Down Syndrome) 1:700 { Other rare ones (7, 9, 12, 14, & 22) Aneuploidy Examples w Human Sex Determining Chromosomes 1. -

Prenatal Profile of Pallister‐Killian Syndrome
Received: 15 May 2018 Revised: 23 June 2018 Accepted: 14 July 2018 DOI: 10.1002/ajmg.a.40499 RESEARCH ARTICLE Prenatal profile of Pallister-Killian syndrome: Retrospective analysis of 114 pregnancies, literature review and approach to prenatal diagnosis E Salzano1 | SE Raible1 | M Kaur1 | A Wilkens2 | G Sperti3 | RK Tilton1 | LR Bettini4 | A Rocca3 | G Cocchi3 | A Selicorni5 | LK Conlin2,6 | D McEldrew2 | R Gupta7 | S Thakur8 | K Izumi1,2,9 | ID Krantz1,9 1Division of Human Genetics, Department of Pediatrics, The Children's Hospital of Philadelphia, Philadelphia, Pennsylvania 2Division of Genomic Diagnostics, Department of Pathology and Laboratory Medicine, The Children's Hospital of Philadelphia, Philadelphia, Pennsylvania 3Neonatology Unit, St. Orsola-Malpighi Polyclinic, Department of Medical and Surgical Sciences (DIMEC), University of Bologna, Bologna, Italy 4Dipartimento di Scienze Della Salute, San Paolo Hospital Medical School, Università degli Studi di Milano, Milan, Italy 5UOC Pediatria, ASST Lariana, Como, Italy 6Department of Pathology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania 7Department of Fetal Medicine, Maharaja Agrasen Hospital, Delhi & Sonepat Genetic and Fetal Medicine Centre, Sonepat, Haryana 8Department of Genetic & Fetal Medicine, Fortis Hospital, Delhi-NCR & Apollo Hospital, Delhi, India 9Department of Pediatrics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania Correspondence Pallister-Killian syndrome (PKS) is a tissue limited mosaic disorder, characterized by variable degrees Krantz ID, Division of Human Genetics, Department of Pediatrics, The Children's of neurodevelopmental delay and intellectual disability, typical craniofacial findings, skin pigmenta- Hospital of Philadelphia, 1007A Abramson tion anomalies and multiple congenital malformations. The wide phenotypic spectrum of PKS in Research Center, 3615 Civic Center conjunction with the mosaic distribution of the i(12p) makes PKS an underdiagnosed disorder.