Photochemical Transformations of Pyridinium Salts: Mechanistic Studies and Applications in Synthesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pyrylium Salt Chemistry Emerging As a Powerful Approach for the Cite This: Chem
Chemical Science View Article Online PERSPECTIVE View Journal | View Issue Over one century after discovery: pyrylium salt chemistry emerging as a powerful approach for the Cite this: Chem. Sci., 2020, 11, 12249 All publication charges for this article construction of complex macrocycles and metallo- have been paid for by the Royal Society of Chemistry supramolecules Yiming Li, ab Heng Wanga and Xiaopeng Li *a Over one century after its discovery, pyrylium salt chemistry has been extensively applied in preparing light emitters, photocatalysts, and sensitizers. In most of these studies, pyrylium salts acted as versatile precursors for the preparation of small molecules (such as furan, pyridines, phosphines, pyridinium salts, thiopyryliums and betaine dyes) and poly(pyridinium salt)s. In recent decades, pyrylium salt chemistry has emerged as a powerful approach for constructing complex macrocycles and metallo-supramolecules. In this perspective, we attempt to summarize the representative efforts of synthesizing and self-assembling Received 20th August 2020 large, complex architectures using pyrylium salt chemistry. We believe that this perspective not only Creative Commons Attribution-NonCommercial 3.0 Unported Licence. Accepted 13th October 2020 highlights the recent achievements in pyrylium salt chemistry, but also inspires us to revisit this chemistry DOI: 10.1039/d0sc04585c to design and construct macrocycles and metallo-supramolecules with increasing complexity and rsc.li/chemical-science desired function. 1. Introduction pyrylium salt with perchlorate as the counterion was reported in 1911 by Baeyer;7 however, since the discovery of pyrylium salts, Pyrylium salts are a type of six-membered cationic heterocycles such salts have been underappreciated for about a half-century. -

From Pyrylium to Pyridinium Salts: Understanding Physicochemical Features
From Pyrylium to Pyridinium Salts: Understanding Physicochemical Features Antonio Franconetti, Lidia Contreras-Bernal and Francisca Cabrera-Escribano Departamento de Química Orgánica, Facultad de Química, Universidad de Sevilla, Apartado de Correos No. 1203, 41071 Sevilla, Spain. Fax: +34954624960; Tel: +34954556868; E-mail: [email protected] Abstract It is known that physical properties of pyridinium salts can be modulated by their chemical characteristics. Owing to their broad range of applications in biology, biotechnology and chemical research, the design and synthesis of a series of 2,4,6-tri- arylpyridinium salts with modified structural backbone have been carried out. The main strands of work have included modifications on pyrylium cation and nature of amines used as precursors. For pyrylium moiety electronic and steric changes in ortho and para positions were performed. The steric influence of the neighbouring groups into the amine reactivity and the changes of the nucleophile strength of this function have also been investigated. Thus, we have analyzed all these aspects to understand the chemical reactivity and seeking further applications. Keywords Pyridinium salts; Pyrylium salts; Amines; Carbohydrates Introduction Pyridinium salts are widely applied as synthetic building blocks to obtain substituted pyridine, dihydropyridine or piperidine. This synthetic strategy has been described for different applications such as total synthesis of Geissoschizine,1 cannabisativine, Lepadin B, among others. Pyridinium salts have been also used to achieve asymmetric and regioselective synthesis by additions of Grignard reagents.2 Furthermore, pyridinium salts have been applied as acylating agents, phase transfer catalyst or ionic liquid and dyes as a result of its intrinsic fluorescence. More recently, bispyridinium moieties have been employed in a tandem cyclization for the synthesis of indolizine derivatives.3 Different methods are available for the synthesis of pyridinium salts depending on the symmetry around of cation. -

Recent Applications of Isatin in the Synthesis of Organic Compounds
The Free Internet Journal Review for Organic Chemistry Archive for Arkivoc 2017, part i, 148-201 Organic Chemistry Recent applications of isatin in the synthesis of organic compounds Razieh Moradi,a Ghodsi Mohammadi Ziarani,*a and Negar Lashgari b a Department of Chemistry, Alzahra University, Tehran, Iran b School of Chemistry, College of Science, University of Tehran, Tehran, Iran E-mail: [email protected] [email protected] Received 12-06-2016 Accepted 02-25-2017 Published on line 04-10-2017 Abstract Isatin has been used in design and synthesis of diverse types of heterocyclic and carbocyclic compounds and considered as a valuable building block in organic synthesis. There is a diversity of multicomponent reactions of this useful reagent. This article aims to review the advances in the use of isatin as starting material in the synthesis of various organic compounds and drugs up to June 2016. Keywords: Isatin, organic compounds, heterocyclic compounds, carbocyclic compounds DOI: https://doi.org/10.24820/ark.5550190.p009.980 Page 148 ©ARKAT USA, Inc Arkivoc 2017, i, 148-201 Moradi, R. et al. Table of Contents 1. Introduction 2. Reactions at the C-3 Position of Isatin 3. Synthesis of Isatin-based Spiro-fused Heterocyclic Frameworks 3.1 Synthesis involving two-component reactions of isatins 3.1.1 Three-membered heterocycles 3.1.2 Five-membered heterocycles 3.1.3 Six-membered heterocycles 3.1.4 Seven-membered heterocycles 4. Synthesis Involving Multicomponent Reactions 4.1 Three-membered heterocycles 4.2 Five-membered heterocycles 4.3 Six-membered heterocycles 4.4 Seven-membered heterocycles 5. -

Novel Derivatives of Nicotinamide Adenine Dinucleotide (NAD) and Their Biological Evaluation Against NAD- Consuming Enzymes
Novel derivatives of nicotinamide adenine dinucleotide (NAD) and their biological evaluation against NAD- Consuming Enzymes Giulia Pergolizzi University of East Anglia School of Pharmacy Thesis submitted for the degree of Doctor of Philosophy July, 2012 © This copy of the thesis has been supplied on condition that anyone who consults it is understood to recognise that its copyright rests with the author and that use of any information derived there from must be in accordance with current UK Copyright Law. In addition, any quotation or extract must include full attribution. ABSTRACT Nicotinamide adenine dinucleotide (β-NAD+) is a primary metabolite involved in fundamental biological processes. Its molecular structure with characteristic functional groups, such as the quaternary nitrogen of the nicotinamide ring, and the two high- energy pyrophosphate and nicotinamide N-glycosidic bonds, allows it to undergo different reactions depending on the reactive moiety. Well known as a redox substrate owing to the redox properties of the nicotinamide ring, β-NAD+ is also fundamental as a substrate of NAD+-consuming enzymes that cleave either high-energy bonds to catalyse their reactions. In this study, a panel of novel adenine-modified NAD+ derivatives was synthesized and biologically evaluated against different NAD+-consuming enzymes. The synthesis of NAD+ derivatives, modified in position 2, 6 or 8 of the adenine ring with aryl/heteroaryl groups, was accomplished by Suzuki-Miyaura cross-couplings. Their biological activity as inhibitors and/or non-natural substrates was assessed against a selected range of NAD+-consuming enzymes. The fluorescence of 8-aryl/heteroaryl NAD+ derivatives allowed their use as biochemical probes for the development of continuous biochemical assays to monitor NAD+-consuming enzyme activities. -

Young Career Focus: Dr. Stephen Thomas (University of Edinburgh, UK)
A166 Synform Young Career Focus Young Career Focus: Dr. Stephen Thomas (University of Edinburgh, UK) Background and Purpose. SYNFORM regularly meets young up-and-coming researchers who are performing exceptionally well in the arena of organic chemistry and related fields of research, in order to introduce them to the readership. This Young Career Focus presents Dr. Stephen Thomas (University of Edinburgh, UK). Biographical Sketch INTERVIEW Stephen Thomas was born in SYNFORM What is the focus of your current research Toronto, Canada, and moved to activity? Somerset (UK) at a young age where he completed his secondary Dr. S. Thomas We are interested in developing and under- school education at Court Fields standing sustainable catalytic methods. Our focus has been Com munity School and Richard on the application and use of the most abundant elements in Huish College. After gaining his the earth’s crust as catalysts the reductive functionalisation undergraduate degree from Cardiff of unsaturated groups. A key driver for us is understanding University (UK), Stephen complet- the methods we develop and how the unique reactivities of ed his PhD with Dr Stuart Warren at first-row transition metals and main group elements can be Dr. Stephen Thomas the University of Cambridge (UK). applied in new ways. Following post-doctoral research with Prof. Andreas Pfaltz at the University of Basel (Switzer- SYNFORM When did you get interested in synthesis? land), Stephen was appointed to a fixed-term lectureship at the University of Bristol (UK) associated with Prof. Varinder Dr. S. Thomas As an undergraduate I was lucky enough to Aggarwal FRS, allowing him to begin his independent join Prof. -

Approaches Towards the Synthesis of Bradyoxetin
University of Mississippi eGrove Electronic Theses and Dissertations Graduate School 2010 Approaches Towards the Synthesis of Bradyoxetin Vanildo Martins Lima Braga Follow this and additional works at: https://egrove.olemiss.edu/etd Part of the Organic Chemistry Commons Recommended Citation Braga, Vanildo Martins Lima, "Approaches Towards the Synthesis of Bradyoxetin" (2010). Electronic Theses and Dissertations. 61. https://egrove.olemiss.edu/etd/61 This Dissertation is brought to you for free and open access by the Graduate School at eGrove. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of eGrove. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Vanildo Martins L. Braga entitled “Approaches Towards the Synthesis of Bradyoxetin. ” I have examined the final copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Pharmaceutical Sciences , with an emphasis in Medicinal Chemistry . ______________________________ John M. Rimoldi, Major Professor Professor of Medical Chemistry We have read this dissertation and recommend its acceptance: _____________________________________ Mitchell A. Avery Professor of Medicinal Chemistry _____________________________________ Franck E. Dayan Adjunct Professor of Medicinal Chemistry _____________________________________ Marc Slattery Professor of Pharmacognosy Accepted for the Council: ____________________________ Dean of the Graduate School STATEMENT OF PERMISSION TO USE In presenting this thesis in partial fulfillment of the requirements for a Ph.D. degree at The University of Mississippi, I agree that the Library shall make it available to borrowers under rules of the Library. -

Recyclization Reactions of 8,10-Dibromocamphor with Grignard and Organolithium Compounds Ihor S
FRENCH-UKRAINIAN JOURNAL OF CHEMISTRY (2021, VOLUME 09, ISSUE 01) Recyclization reactions of 8,10-dibromocamphor with Grignard and organolithium compounds Ihor S. Verenka, Marian V. Gorichko* Department of Chemistry, Taras Shevchenko National University of Kyiv, Volodymyrska Street, 64/13, Kyiv 01601, Ukraine [email protected] Keywords: camphor, recyclization, Grob fragmentation, intramolecular alkylation, bicyclo[3.2.0]heptane. Grignard reagents and organolithium compounds react with 8,10-dibromocamphor to afford substituted 1-methyl-2-methylenebicyclo[3.2.0]heptanes. Recyclization proceeds via intramolecular enolate alkylation and Grob fragmentation of the reaction intermediates. All compounds have been characterized by 1H, 13C and 19F NMR spectroscopy and their chemical composition proved by HRMS analyses. The relative spatial arrangement of substituents in the molecule of (1-methyl-2- methylenebicyclo[3.2.0]heptan-6-yl)diphenylmethanol was studied by NOESY experiments. ________________________________________________________________________________ Introduction Even nowadays, comparing to other Camphor is known as the naturally relatively simple natural molecules, the available starting material in a number of chemistry of camphor has many uncharted syntheses of more complex compounds from the domains. middle of the XIX century. Nowadays many Electrophilic bromination is the very syntheses of steroids [1-3], terpenoids [4-12], important approach in the preparation of bromo- vitamins [13-15] and other natural products [16- functionalized terpenoid derivatives, which are 19] that involve camphor or its derivatives are common as intermediates in organic synthesis described in the literature. because of the ease of further modifications via Camphor derived chiral auxiliaries can nucleophilic substitutions or various skeletal be prepared in both enantiomeric forms since rearrangements and cyclizations [26–30]. -

Heterocyclic Chemistrychemistry
HeterocyclicHeterocyclic ChemistryChemistry Professor J. Stephen Clark Room C4-04 Email: [email protected] 2011 –2012 1 http://www.chem.gla.ac.uk/staff/stephenc/UndergraduateTeaching.html Recommended Reading • Heterocyclic Chemistry – J. A. Joule, K. Mills and G. F. Smith • Heterocyclic Chemistry (Oxford Primer Series) – T. Gilchrist • Aromatic Heterocyclic Chemistry – D. T. Davies 2 Course Summary Introduction • Definition of terms and classification of heterocycles • Functional group chemistry: imines, enamines, acetals, enols, and sulfur-containing groups Intermediates used for the construction of aromatic heterocycles • Synthesis of aromatic heterocycles • Carbon–heteroatom bond formation and choice of oxidation state • Examples of commonly used strategies for heterocycle synthesis Pyridines • General properties, electronic structure • Synthesis of pyridines • Electrophilic substitution of pyridines • Nucleophilic substitution of pyridines • Metallation of pyridines Pyridine derivatives • Structure and reactivity of oxy-pyridines, alkyl pyridines, pyridinium salts, and pyridine N-oxides Quinolines and isoquinolines • General properties and reactivity compared to pyridine • Electrophilic and nucleophilic substitution quinolines and isoquinolines 3 • General methods used for the synthesis of quinolines and isoquinolines Course Summary (cont) Five-membered aromatic heterocycles • General properties, structure and reactivity of pyrroles, furans and thiophenes • Methods and strategies for the synthesis of five-membered heteroaromatics -
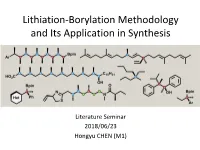
Lithiation-Borylation Methodology and Its Application in Synthesis
Lithiation-Borylation Methodology and Its Application in Synthesis Literature Seminar 2018/06/23 Hongyu CHEN (M1) Contents • Introduction • Part 1 : Factors responsible for the 1,2-migration • Part 2 : Factors responsible for stereocontrol • Part 3 : Application of lithiation-borylation reaction in synthesis • Summary Contents • Introduction • Part 1 : Factors responsible for the 1,2-migration • Part 2 : Factors responsible for stereocontrol • Part 3 : Application of lithiation-borylation reaction in synthesis • Summary Anatomy of The Lithiation-Borylation Reaction R3 B(OR)2 Stereoretentive B(OR)2 R2 R Borylation 2 1,2-Metallate R3 OLG R1 R1 Rearrangement R Homologated X Li 3 Boronate complex B(OR)2 product R2 R2 OLG Lithiation OLG R1 R1 OLG = OCb or OTIB Lithium carbenoid R2 X = H or SnR OLG R2 3 Stereoinvertive R 1 R3 R1 Borylation B(OR)2 1,2-Metallate R B(OR)2 3 Rearrangement Boronate complex Homologated product ・ R1, R2 and R3 = Alkyl, Alkenyl or Aryl ・ Reagent Control ・ Complete Stereospecificity Arylation ・ Contiguous Stereocenters Alkynylation ・ Quaternary Stereocenters Oxidation Fluorination ・ Natural Product Synthesis Assembly-Line Synthesis ・ Assembly-Line Synthesis http://www.chm.bris.ac.uk/org/aggarwal/research.php#li-b The First Nonenzymatic Asymmetric Synthesis (H. C. Brown, 1961) DG )2BH [O] + BH3 )2B 0 ℃ H HO H 90% yield, 87% ee cis, cis; cis, trans; trans, trans etc. R*CHCHCHCHR’ R*OH R* R' R*CO2H R* R’ R' R*D R*CHO General Asymmetric Synthesis R* R* R' R*CH OH via Chiral Organoboranes 2 R*NH2 R*CH2CHCH2 R* B R*R’NH R*R’CHNH2 R*CCR’ R*CCH R*COR’ R*CH2COR’ R*R’CHOH R*CH2CN R*COCCR’ R*CH2CO2Et R*R ’COH Brown, H. -

1 the Principle of Conservation of Structural Aspect: Facilitating Visual Communication and Learning in Organic Chemistry Instr
1 The Principle of Conservation of Structural Aspect: Facilitating Visual Communication and Learning in Organic Chemistry Instruction John Andraos CareerChem 504-1129 Don Mills Road Toronto, ON M3B 2W4 Canada [email protected] Abstract An effective pedagogical method is presented for the visual communication of chemical reactions learned in organic chemistry undergraduate courses. The basis for the method is the preservation of the visual aspect of reactant and product structures so that the tracking of cleaved and formed chemical bonds is made self-evident. This consequently leads to improved clarity of presentation and a better understanding and grasp of proposed reaction mechanisms to explain product outcomes. The method is demonstrated for a variety of individual reaction types and synthesis plans. Various visual training exercises are also presented using ChemDraw Ultra 7.0 software and literature table of contents (TOC) graphics appearing in journal articles. Keywords: upper-division undergraduate, curriculum, organic chemistry pedagogy, problem solving, organic synthesis Introduction It is well known that when students encounter organic chemistry for the first time in undergraduate courses, they perceive the subject as “hard”. From their point of view their complaint stems from two major issues: (a) the apparent overwhelming volume of material that needs to be digested and learned in a relatively short period of time 2 (typically a three-month course session); and (b) the material is presented in a visual manner that is not always “easy” or evident to understand what is going in any given chemical reaction that is presented to them. The inevitable outcome is increased frustration and ultimately a dislike for the subject for the majority of students, particularly those that take organic chemistry as a standard mandatory requirement for other disciplines such as pre-medical studies and life sciences. -

Versatile Synthetic Methods for Photoluminescent Pyrylium Tosylates Jung Jae Koh University of Nevada, Las Vegas, [email protected]
UNLV Theses, Dissertations, Professional Papers, and Capstones 8-1-2015 Versatile Synthetic Methods for Photoluminescent Pyrylium Tosylates Jung Jae Koh University of Nevada, Las Vegas, [email protected] Follow this and additional works at: https://digitalscholarship.unlv.edu/thesesdissertations Part of the Chemistry Commons Repository Citation Koh, Jung Jae, "Versatile Synthetic Methods for Photoluminescent Pyrylium Tosylates" (2015). UNLV Theses, Dissertations, Professional Papers, and Capstones. 2487. https://digitalscholarship.unlv.edu/thesesdissertations/2487 This Thesis is brought to you for free and open access by Digital Scholarship@UNLV. It has been accepted for inclusion in UNLV Theses, Dissertations, Professional Papers, and Capstones by an authorized administrator of Digital Scholarship@UNLV. For more information, please contact [email protected]. VERSATILE SYNTHETIC METHODS FOR PHOTOLUMINESCENT PYRYLIUM TOSYLATES by Jung Jae Koh Bachelor of Biochemistry University of Nevada, Las Vegas 2011 A thesis submitted in partial fulfillment of the requirements for the Master of Science - Chemistry Department of Chemistry and Biochemistry College of Science The Graduate College University of Nevada, Las Vegas August 2015 Copyright by Jung Jae Koh, 2015 All Rights Reserved Thesis Approval The Graduate College The University of Nevada, Las Vegas July 21, 2015 This thesis prepared by Jung Jae Koh entitled Versatile Synthetic Methods for Photoluminescent Pyrylium Tosylates is approved in partial fulfillment of the requirements for the degree of Master of Science – Chemistry Department of Chemistry and Biochemistry Pradip K. Bhowmik, Ph.D. Kathryn Hausbeck Korgan, Ph.D. Examination Committee Co-Chair Graduate College Interim Dean Vernon F. Hodge, Ph.D. Examination Committee Co-Chair Dong-Chan Lee, Ph.D. -

Pyrylium Salts with Long Alkyl Substituents, II
Pyrylium Salts with Long Alkyl Substituents, II [1] 2,4-Dimethyl-6-undecylpyrylium Perchlorate and Derived Pyridinium Salts Mariana Bogatian, Calin Deleanu, Gheorghe Mihai, and Teodor Silviu Balaban* Center of Organic Chemistry of the Roumanian Academy Splaiul Independentei 202 B, 71141 Bucharest, PO 15/258, Roumania Z. Naturforsch. 47b, 1011-1015 (1992); received September 25, 1991/January 10, 1992 Pyrylium Salts, Pyridinium Salts, Synthetic Amphiphiles, NMR Spectra The crystalline title pyrylium salt was obtained by SnCl4 catalysed acylation of mesityl oxide with lauroyl chloride followed by treatment with perchloric acid and column chromatography. This new pyrylium salt was converted in high yields into the corresponding pyridine and N-substituted pyridinium salts (N-methyl, N-phenyl, N-(4-«-butylphenyl), and N-dodecyl) whose proton (300 MHz) and carbon-13 (75 MHz) NMR spectra are presented. Introduction eleven carbon a-alkyl side-chain and pyridinium One long aliphatic (lipophilic) chain attached to salts derived therefrom. strongly polar (hydrophilic) groups leads to com pounds with tensioactive properties. These are due either to micelar aggregates or to membranes. Two Results and Discussion long lipophilic chains attached to one polar group The acylation of mesityl oxide (1) was effected lead to amphiphiles, which can form bilayer mem with lauroyl chloride of comercial source, by branes [2]. Some such synthetic analogs of bio known methods [ 8], When A1C1 3 was used as acy- membranes have been prepared and their catalytic lating catalyst, the yields were low (<5% ) due to activity has been investigated and compared with the poor isolation of the desired title salt 2, while that of phospholipids and lecithins [3].