Cleaning, Disinfection and Sterilization Guide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Usefulness of the Harmonic Scalpel in Thyroid Surgery
ORIGINAL ISSN: 2005-162X J Korean Thyroid Assoc 2012 November 5(2): 138-142 ARTICLE http://dx.doi.org/10.11106/jkta.2012.5.2.138 Usefulness of the Harmonic Scalpel in Thyroid Surgery Hwan Choe, Kwang-Yoon Jung, Soon-Young Kwon, Jeong-Soo Woo, Min Woo Park and Seung-Kuk Baek Department of Otolaryngology-Head and Neck Surgery, Korea University College of Medicine, Seoul, Korea Background and Objectives: The harmonic scalpel using the ultrasonic energy is able to grasp and divide tissue while sealing small vessels in narrow operating fields. The aim of the present study was to evaluate the usefulness of the harmonic scalpel in thyroid surgery. Materials and Methods: This study was performed for 247 patients who underwent thyroidectomy. According to the use of harmonic Scalpel, the patients could be divided into two groups: the conventional technique (CT) group of knot tying and the harmonic scalpel (HS) group. Results: For hemithyroidectomy, operation time and hospital stay were shorter in the HS group compared with the CT group (p<0.05). For total thyroidectomy with central neck dissection (CND), operation time, total drainage volume, drain removal date, and hospital stay were significantly reduced in the HS group (p<0.05). Among the patients who underwent total thyroidectomy with CND with the HS, one patient (2.9%) showed transient recurrent laryngeal nerve palsy. Transient hypoparathyroidism showed significantly lower incidence in the HS group (p<0.05). Conclusion: HS might be cost-effective by reducing operation time and hospital stay -

The Basic Surgery Kit
GLOBAL EXCLUSIVE > SURGERY > PEER REVIEWED The Basic Surgery Kit Jan Janovec, MVDr, MRCVS VRCC Veterinary Referrals Laurent Findji, DMV, MS, MRCVS, DECVS Fitzpatrick Referrals Considering the virtually limitless range of surgical instruments, it can be difficult to assemble a cost-effective basic surgery kit. Some instruments may misleadingly appear multipurpose, but their misuse may damage them, leading to unnecessary replacement costs or, worse, intraoperative accidents putting the patient’s safety at risk. Many instru- ments are available in different qualities and materials (eg, tungsten carbide instruments— more expensive but much more resistant to wear and corrosion than stainless steel) and Minimal Basic Surgery Kit varied sizes to match the purpose of their use as well as the size of the surgeon’s hand. n 1 instrument case Cutting Instruments n 1 scalpel handle Scalpel n 1 pair Mayo scissors The scalpel is an indispensible item in a surgical kit designed to make sharp incisions. Scalpel incision is the least traumatic way of dissection, but provides no hemostasis. n 1 pair Metzenbaum scissors Scalpel handles come in various sizes, each accommodating a range of disposable n 1 pair suture scissors blades (Figure 1). Entirely disposable scalpels are also available. n 1 pair Mayo-Hegar needle holder Scissors n 1 pair Brown-Adson tissue forceps Scissors are used for cutting, albeit with some crushing effect, and for blunt dissection. n 1 pair DeBakey tissue forceps Fine scissors, such as Metzenbaum scissors (Figure 2), should be reserved for cutting n 4 pairs mosquito hemostatic forceps and dissecting delicate tissues. Sturdier scissors, such as Mayo or suture scissors, are designed for use on denser tissues (eg, fascia) or inanimate objects (eg, sutures, drapes). -

Covidien-Or-Products-Catalog.Pdf
OR Products 1-800-962-9888 1-800-962-9888 The products shown in this catalog may not be licensed or registered in all parts of the world. Please contact your sales representative for availability. 1 Table of Contents OR Suction OR Safety Argyle™ Yankauer Suction Instruments, Rigid Type ................ 2 Sponge Counting Systems ........................................................ 18 Argyle™ Yankauer Suction Instruments, Flexible Type ............ 3 Solidification Products .............................................................. 18 Argyle™ Poole and Suction Sets ................................................... 4 Devon™ Hands Free Transfer Magnetic Drapes ..................... 19 Curity™ Suction Instruments and Sets ........................................ 4 Devon™ Magnetic Drapes with Neutral Zones ....................... 19 Argyle™ Suction Tubing ................................................................ 5 Devon™ Trays and Basins .......................................................... 19 ™ Argyle™ Universal Bubble Tubing ............................................... 6 Devon Reusable Magnetic Drapes ......................................... 20 ™ Argyle™ Tubing Connectors ......................................................... 6 Devon Blade Shield Scalpel Holder ....................................... 20 ™ Solidification Products ................................................................. 7 Devon Magnetic Needle Finder ............................................. 21 Devon™ Safe-T-Strainer Instrument Handling -

Otology – Otoscopy
EXTRACT FROM THE ENT CATALOG OTOLOGY – OTOSCOPY 10th EDITION 1/2019 It is recommended to check the suitability of the product for the intended procedure prior to use. Please note that the described products in this medium may not be available yet in all countries due to different regulatory requirements. Not all the products listed in this document are certified according to Regulation 2017/745/EU. For this reason, some products requiring certification under this Regulation may not be available in every country. © All product illustrations, product descriptions and texts are the intellectual property of KARL STORZ SE & Co. KG. Their use and reproduction by third parties require the express approval of KARL STORZ SE & Co. KG. All rights reserved. 12-20 OTOLOGY – OTOSCOPY OTOSCOPY . .2-7 INSTRUMENTS FOR DIAGNOSIS AND THERAPY – OUTER EAR . .8-23 EAR MICROSURGERY . .24-75 ENDOSCOPIC MIDDLE EAR SURGERY . .76-82 RACKS AND CONTAINERS . .83-86 Otoscopy with HOPKINS® Telescopes Special Features: ● For visualization of the eardrum ● HOPKINS® telescopes available in diameters 1.9 mm, 2.7 mm, 3 mm and 4 mm for ear diagnosis and surgery 2 2-94 2 OTO-TEL Otoscopy with HOPKINS® Telescopes Diameter 1.9 mm, length 10 cm For preoperative and postoperative examinations of the middle ear 1232 AA 1232 AA HOPKINS® Straight Forward Telescope 0°, diameter 1.9 mm, length 10 cm, autoclavable, fiber optic light transmission incorporated, color code: green 1232 BA HOPKINS® Forward-Oblique Telescope 30°, diameter 1.9 mm, length 10 cm, autoclavable, fiber optic light -

What to Teach a New Technician: Part II
INSTRUMENT WHISPERER What to Teach a New Technician: Part II BY RICK SCHULTZ he Instrument Whisperer retractor is used to move tissue, muscle column in the May/June issue and organs aside in order to expose of PROCESS addressed the the surgical site. It is important for the importance of process in the technician to know the proper names and Thiring, training and retention of quality how to measure (See Figure A) to ensure Sterile Processing (SP) professionals. It the correct retractor is placed into the also specifically addressed the importance surgical set. of instrument inspection and the new technician’s need to know the basic instrument groups. That article focused on inspection of scissors, needle holders and hemostats. This article focuses on Figure B the following three instrument groups: • Retractors; • Suction devices; and • Scalpel handles Note: Once the right person has been hired, it is beneficial to have them observe four or five surgeries of various specialties, so they can see the use of the products they will be putting into trays. Figure A Retractors Retractors play a crucial role in surgery. Two common types of hand-held As with all surgical instruments, it is retractors are the Kelly retractor important to learn the most commonly- (Figure B) and the Richardson retractor used retractors, their main differences (Figure C). and how to properly inspect them. The difference between these retractors There are several broad categories of is the depth and width of the blades. A Figure C retractors: Kelly retractor is always larger than a • Hand-held retractors – A hand-held Richardson retractor. -

VATS Instruments
VATS Instruments Video-Assisted Thoracoscopic Surgery Surgical Speciality Products VATS Instruments Video-Assisted Thoracoscopic Surgery Forceps / Clamps | Diameter 7 mm | Usable length 25 cm STANDARD INSTRUMENT - COMPLETE Description FOERSTER LUNG GRASPER 25 cm 15 mm oval ring jaws, 15° angled, 63 mm jaw length, Forceps/Clamps double action ¹⁄1 FC810R 25 cm TISSUE GRASPING FORCEPS 30° angled, 65 mm jaw length, single action ¹⁄1 FC811R 25 cm MARYLAND DISSECTING FORCEPS curved, 21 mm jaw length, double action ¹⁄1 FC812R 25 cm DISSECTING FORCEPS straight, 25 mm jaw length, double action ¹⁄1 FC813R 2 SINGLE COMPONENTS Jaw Insert Insulated outer tube Handle Forceps/Clamps ½ FC860R without ratchet ½ FC800R FC861R FC808P with ratchet, disengageable ½ FC801R FC862R ½ FC863R 3 VATS Instruments Video-Assisted Thoracoscopic Surgery Forceps / Clamps | Diameter 7 mm | Usable length 25 cm STANDARD INSTRUMENT - COMPLETE Description OVERHOLT DISSECTING FORCEPS 90° 65 mm jaw length, double action Forceps/Clamps 25 cm ¹⁄1 FC814R OVERHOLT DISSECTING FORCEPS 90° delicate, 64 mm jaw length, double action 25 cm ¹⁄1 FC815R 25 cm OVERHOLT DISSECTING FORCEPS 60° 40 mm jaw length, double action ¹⁄1 FC816R BIOPSY FORCEPS 30° curved, 14 mm jaw length, double action ¹⁄1 FC820R 4 SINGLE COMPONENTS Jaw Insert Insulated outer tube Handle Forceps/Clamps ½ FC864R without ratchet ½ FC800R FC865R FC808P with ratchet, disengageable ½ FC801R FC866R ½ FC870R 5 VATS Instruments Video-Assisted Thoracoscopic Surgery Forceps / Clamps | Diameter 7 mm | Usable length 25 cm STANDARD -

THERMAL SCALPEL SYSTEM MODEL PRECISION 8400 (P8400) HANDLE Part Number 7013-8050
THERMAL SCALPEL SYSTEM MODEL PRECISION 8400 (P8400) HANDLE Part Number 7013-8050 For use with the Hemostatix Thermal Scalpel System Model P8400 Controller OPERATING MANUAL INTRODUCTION HEMOSTATIX THERMAL SCALPEL BLADES are similar in size, shape, and sharpness to traditional steel scalpel blades; however, Hemostatix blades can be heated to a user- selected temperature appropriate for sealing small vessels as they are cut. To cut and simultaneously seal blood vessels effectively with minimum tissue damage, the sharp steel cutting edge of a heated scalpel blade must be uniformly maintained at the desired temperature within narrow limits. The Hemostatix Thermal Scalpel System utilizes micro-circuitry incorporated within the blade itself to maintain the cutting edge temperature within the necessary tolerance, selectively delivering additional thermal energy only to those regions of the blade using heat due to tissue contact. By so doing, the Hemostatix Thermal Scalpel System automatically compensates for the varying degrees of heat loss that occur during surgical procedures (depending on the type of tissue being incised and the rate at which cutting is carried out), maintaining the cutting edge in the desired temperature range. In contrast with electrosurgical devices, the Hemostatix Thermal Scalpel System passes no electrical current through the patient, and there is no sparking or electrical arcing to the tissue. Electrosurgical devices “cut” and/or cauterize using electrical currents which pass through the patient vaporizing tissue at the point of contact and generating heat and tissue damage down the path of the electrical current. The Hemostatix Thermal Scalpel System cuts tissue with a sharpened steel edge, like a conventional cold-steel scalpel blade, and simultaneously seals blood vessels using heat thermally conducted to the tissue from an elevated-temperature blade which is electrically insulated from the patient. -

National Institute for Health and Clinical Excellence
242 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedures overview of tonsillectomy using ultrasonic scalpel Introduction This overview has been prepared to assist members of the Interventional Procedures Advisory Committee (IPAC) in making recommendations about the safety and efficacy of an interventional procedure. It is based on a rapid review of the medical literature and specialist opinion. It should not be regarded as a definitive assessment of the procedure. Date prepared This overview was prepared in August 2005. Procedure names • Harmonic scalpel for tonsillectomy. • Ultrasonic scalpel. • Ultrasound activated scalpel. Specialty society British Association of Otorhinolaryngologists, Head and Neck Surgeons. Description Indications Indications for tonsillectomy include recurrent acute or chronic tonsillitis, peritonsillar abscess and pharyngeal obstruction/obstructive sleep apnoea. Life-threatening complications of these conditions are rare and the main aim of surgery is to relieve symptoms. Current treatment and alternatives Surgical removal of the tonsils (tonsillectomy) is one of the most common surgical procedures in the UK. The operation is performed in many different ways but can be broadly divided into two stages: removal (either complete or partial) of the tonsil, followed by control of bleeding (haemostasis). Traditional ‘cold steel’ tonsillectomy consists of two stages: removal of the tonsil followed by haemostasis. Bleeding is controlled by pressure, then by ligatures. The use of ligatures may be supplemented by diathermy and the use of packs. Diathermy uses radiofrequency energy applied directly to the tissue, and can be bipolar (current passes between the two tips of the forceps) or monopolar (current passes between the forceps tips and a plate attached to the patient’s skin). -

3544-3551-Study on the Use of Focus Harmonic Scalpel in Thyroidectomies
Eur opean Rev iew for Med ical and Pharmacol ogical Sci ences 2016; 20: 3544-3551 Study on the use of focus harmonic scalpel in thyroidectomies: is it useful also in preserving voice function? A. MINNI 1, D. ROSATI 1, C. CAVALIERE 1, A. DE CARLO 1, G. ILLUMINATI 2, V. SCARANO CATANZARO 2, M. BONONI 3 1Department of Sensory Organs, “Sapienza” University of Rome, Azienda Policlinico Umberto I, Rome, Italy 2“F. Durante” Department of Surgical Science, “Sapienza” University of Rome, Azienda Policlinico Umberto I, Rome, Italy 3Department of Surgery “P. Valdoni”, “Sapienza” University of Rome, Azienda Policlinico Umberto I, Rome, Italy Abstract. – OBJECTIVE : The aim of this ran - Key Words: domized study is to evaluate the real benefits of Thyroid surgery, Focus Harmonic Scalpel, Voice the FOCUS Harmonic Scalpel in total thyroidec - function . tomies compared with conventional ligation, re - garding operative time, postoperative blood loss, length of stay and complications. Further - Introduction more, as never seen in other studies, we studied the effects of using the FOCUS during thy - roidectomy analyzing the vocal production of During the past decades, the techniques of thy - patients before and after surgery. roid surgery have undergone small changes only. PATIENTS AND METHODS: We enrolled 361 On the other hand, the last twenty years have patients who underwent total thyroidectomy from 2008 to 2014. It was a randomized clinical trial in seen dynamic development of new instruments which all the surgical procedures were performed that has had a significant impact on this kind of by the same surgeon. Patients were randomized surgery 1-3 . -
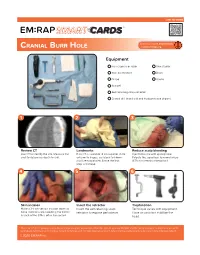
Cranial Burr Hole
SMART LINK TO VIDEO ™ CRANIAL BURR HOLE Equipment Hair clippers or razor Skin stapler Skin disinfectant Drain Drape Gauze Scalpel Self-retaining scalp retractor Cranial drill (hand drill and Hudson-brace shown) 1 2 3 Review CT Landmarks Reduce scalp bleeding Use CT to identify the site. Measure the If no CT is available: 2 cm superior, 2 cm Inject lidocaine with epinephrine. skull to determine depth to drill. anterior to tragus, ipsilateral to blown Palpate the superficial temporal artery pupil (temporal site). Shave the hair, (STA) and remain anterior to it. prep and drape. 4 5 6 Skin incision Insert the retractor Trephination Make a 3-5 cm vertical incision down to Insert the self-retaining scalp Technique varies with equipment. bone. Control scalp bleeding; the frontal retractor to expose periosteum. Have an assistant stabilize the branch of the STA is often transected. head. This is for reference purposes only. Consent to photograph was obtained from the patient or family. EM:RAP and the authors assume no liability for use of the techniques described. Local practice, current guidelines, and clinician experience should determine the exact procedural process in any individual patient. © 2020 EM:RAP Inc. SMART LINK TO VIDEO ™ CRANIAL BURR HOLE 7 A B Using a Hudson-Brace Drilling with the perforator bit Drilling with the conical burr Use the perforator bit to drill When the drilling motion becomes through the outer table, felt as a jagged, switch to the conical burr to smooth drilling motion. trephinate the inner table of the skull. Note: the non-rotating hand should provide counter-torque and resist forward motion of the drill. -

Commonly Used Surgical Instruments
COMMON SURGICAL INSTRUMENTS The operating room contains a multitude of instruments fit for accomplishing a number of procedures. Note that this is not an exhaustive list of instruments, but rather some that you will encounter frequently. SCALPEL Used for initial incision and cutting tissue. Consists of a blade and a handle. Surgeons #10 Blade: Used primarily for #11 Blade: Used for making #15 Blade: Smaller version of often refer to the making large skin incisions, precise or sharply angled #10 blade used for making finer instrument by its blade e.g., in laparotomy. incisions. incisions. number. Pott’s Scissors: Fine scissors used for creating SCISSORS incisions in blood vessels. Used for cutting tissue, suture, or for Iris Scissors: Used Mayo Scissors: Heavy scissors Metzenbaum Scissors: Lighter dissection. Scissors for fine dissection available in multiple varieties. scissors used for cutting delicate can be straight or and cutting fine Straight scissors are used for tissue (e.g., heart) and for blunt curved, and may be suture. Originally cutting suture (“suture scissors”), dissection. Also called “Metz” in for ophthalmic used for cutting heavy while curved scissors are used practice. procedures, but or finer structures. for cutting heavy tissue (e.g., now serves fascia). multipurpose role. Bonney DeBakey Russian Forceps: Forceps: Forceps: Heavy Used for Used for Tissue Forceps: Non-toothed forceps atraumatic atraumatic FORCEPS forceps used for fine handling used for tissue tissue Also known as non- of tissue and traction during holding grasping grasping locking forceps, dissection. thick tissue during during grasping forceps, (e.g., fascial dissection. dissection. thumb forceps, or closure). -
A Comparative Study of the Use of Harmonic Scalpel Versus
[Downloaded free from http://www.nigerianjsurg.com on Thursday, May 18, 2017, IP: 165.255.213.249] Original Article A Comparative Study of the Use of Harmonic Scalpel versus Unipolar Cautery in Modified Radical Mastectomy Pallavi Mittal, Ashwani Kumar, Sunmeet Kaur1, Paras Kumar Pandove, Rachan Lal Singla, Jagpal Singh Department of Surgery, Context: Oncosurgery is an emerging branch with the set goals of prolonging Government Medical College and Rajindra Hospital, the life and ensuring the best possible quality of life to the surviving patient. Patiala, Punjab, 1Medical The use of harmonic scalpel has proved to be beneficial in a variety of surgeries Student, All India Institute but its role in breast surgery is still controversial. Aims: We conducted this of Medical Sciences, Abstract study to compare the intraoperative and postoperative outcomes in modified New Delhi, India radical mastectomy using harmonic scalpel versus electrocautery. Subjects and Methods: Fifty female patients with confirmed diagnosis of breast carcinoma and planned for modified radical mastectomy were taken up for surgery. Twenty‑five patients were operated using harmonic scalpel (Group A) and another 25 were operated using unipolar cautery (Group B). Results: The mean operative time was significantly longer with harmonic scalpel when compared to that with electrocautery (140.40 ± 29.96 vs. 99.80 ± 24.00 min, P < 0.001). The smaller amount of drainage content (431.60 ± 145.94 vs. 594.20 ± 278.63, P = 0.013) and intraoperative blood loss (426.00 ± 76.54 vs. 502.00 ± 104.56, P = 0.005) in the group operated with the ultrasound harmonic scalpel was statistically significant.