(12) Patent Application Publication (10) Pub. No.: US 2017/0073340 A1 Fieldhouse Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

TE INI (19 ) United States (12 ) Patent Application Publication ( 10) Pub
US 20200187851A1TE INI (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No .: US 2020/0187851 A1 Offenbacher et al. (43 ) Pub . Date : Jun . 18 , 2020 ( 54 ) PERIODONTAL DISEASE STRATIFICATION (52 ) U.S. CI. AND USES THEREOF CPC A61B 5/4552 (2013.01 ) ; G16H 20/10 ( 71) Applicant: The University of North Carolina at ( 2018.01) ; A61B 5/7275 ( 2013.01) ; A61B Chapel Hill , Chapel Hill , NC (US ) 5/7264 ( 2013.01 ) ( 72 ) Inventors: Steven Offenbacher, Chapel Hill , NC (US ) ; Thiago Morelli , Durham , NC ( 57 ) ABSTRACT (US ) ; Kevin Lee Moss, Graham , NC ( US ) ; James Douglas Beck , Chapel Described herein are methods of classifying periodontal Hill , NC (US ) patients and individual teeth . For example , disclosed is a method of diagnosing periodontal disease and / or risk of ( 21) Appl. No .: 16 /713,874 tooth loss in a subject that involves classifying teeth into one of 7 classes of periodontal disease. The method can include ( 22 ) Filed : Dec. 13 , 2019 the step of performing a dental examination on a patient and Related U.S. Application Data determining a periodontal profile class ( PPC ) . The method can further include the step of determining for each tooth a ( 60 ) Provisional application No.62 / 780,675 , filed on Dec. Tooth Profile Class ( TPC ) . The PPC and TPC can be used 17 , 2018 together to generate a composite risk score for an individual, which is referred to herein as the Index of Periodontal Risk Publication Classification ( IPR ) . In some embodiments , each stage of the disclosed (51 ) Int. Cl. PPC system is characterized by unique single nucleotide A61B 5/00 ( 2006.01 ) polymorphisms (SNPs ) associated with unique pathways , G16H 20/10 ( 2006.01 ) identifying unique druggable targets for each stage . -

Programa Cooperación Farma-Biotech 8º Encuentro (7 De Mayo De 2013)
Programa Cooperación Farma-Biotech 8º encuentro (7 de mayo de 2013) Lorediplon: “best-in-class” product for insomnia treatment Madrid,,y 7 de mayo de 2013 Programa Cooperación Farma-Biotech 8º encuentro (7 de mayo de 2013) Content 1. The Company 2. The Product a) Target Indications b) Innovative mechanisms of action c) Differential features facing the market d) Current status of development e) IPR protect io n f) Pitfalls & Risks to be considered 3. Partnering Opportunities Programa Cooperación Farma-Biotech 8º encuentro (7 de mayo de 2013) The Company Who we are IItnternati onal pri vat tlely-hhldheld pharmaceu tiltical company Founded in 1959 Mission Business areas To advance the wellbeinggy of society Pharmaceuticals OTCs & personal health Diagnostics Worldwide operations Fine chemicals Headquarter in Barcelona Food & feed additives Presence in 93 countries Programa Cooperación Farma-Biotech 8º encuentro (7 de mayo de 2013) The Company Total turnover 2012 €819 million (+2% vs 2011) International turnover €365 million (44% of total) Total staff 2012 2,201 (abroad 593) Inves tmen t in R&D 12. 1% ofitilf prescription sales Given the maximum rating by the Spanish national pharma assessment system (Profarma) Programa Cooperación Farma-Biotech 8º encuentro (7 de mayo de 2013) The Product - Target Indication Insomnia In 2010, there were 53 million prevalent cases of insomnia in the 7 MM (US, Japan, France, Germany, Italy, Spain, and UK). US (25.7 million), Japan (8.2 million), France (6.8 million), Germany and Italy (3.5 million), Spain and -

Patent Application Publication ( 10 ) Pub . No . : US 2019 / 0192440 A1
US 20190192440A1 (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2019 /0192440 A1 LI (43 ) Pub . Date : Jun . 27 , 2019 ( 54 ) ORAL DRUG DOSAGE FORM COMPRISING Publication Classification DRUG IN THE FORM OF NANOPARTICLES (51 ) Int . CI. A61K 9 / 20 (2006 .01 ) ( 71 ) Applicant: Triastek , Inc. , Nanjing ( CN ) A61K 9 /00 ( 2006 . 01) A61K 31/ 192 ( 2006 .01 ) (72 ) Inventor : Xiaoling LI , Dublin , CA (US ) A61K 9 / 24 ( 2006 .01 ) ( 52 ) U . S . CI. ( 21 ) Appl. No. : 16 /289 ,499 CPC . .. .. A61K 9 /2031 (2013 . 01 ) ; A61K 9 /0065 ( 22 ) Filed : Feb . 28 , 2019 (2013 .01 ) ; A61K 9 / 209 ( 2013 .01 ) ; A61K 9 /2027 ( 2013 .01 ) ; A61K 31/ 192 ( 2013. 01 ) ; Related U . S . Application Data A61K 9 /2072 ( 2013 .01 ) (63 ) Continuation of application No. 16 /028 ,305 , filed on Jul. 5 , 2018 , now Pat . No . 10 , 258 ,575 , which is a (57 ) ABSTRACT continuation of application No . 15 / 173 ,596 , filed on The present disclosure provides a stable solid pharmaceuti Jun . 3 , 2016 . cal dosage form for oral administration . The dosage form (60 ) Provisional application No . 62 /313 ,092 , filed on Mar. includes a substrate that forms at least one compartment and 24 , 2016 , provisional application No . 62 / 296 , 087 , a drug content loaded into the compartment. The dosage filed on Feb . 17 , 2016 , provisional application No . form is so designed that the active pharmaceutical ingredient 62 / 170, 645 , filed on Jun . 3 , 2015 . of the drug content is released in a controlled manner. Patent Application Publication Jun . 27 , 2019 Sheet 1 of 20 US 2019 /0192440 A1 FIG . -

Download English
ASEBIO Report News and trends from the Spanish Biotech Sector and company guide 2015 Edited by Spanish Bioindustry Association (ASEBIO) All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including by photocopies, recordings or any information storage and retrieval, without written permission from the copyright holders. © ASEBIO 1st edition: september 2016 Design and editorial coordination: Cyan, Proyectos Editoriales, S.A. Index 5 The Spanish biotechnology sector in 2015 13 Companies launched in 2015 19 Business activities 29 Industrial property rights and knowledge generation 35 Health – red biotechnology 41 Agrifood – green biotechnology 45 Industrial – white biotechnology 49 Financial environment 53 Internationalisation 59 Biolatam 2015 63 Who is who? – ASEBIO Members 64 Business members 77 Other organisations 1. THE SPANISH BIOTECHNOLOGY SECTOR IN 2015 The Spanish biotechnology 1. sector in 2015 • 628 companies confirmed biotech- • Global industry turnover continued to nology to be their main and/or exclu- grow, rising to 107,788 in 2014 (13.28% sive activity (companies known as bio- more than the previous year). This growth techs), up by 13.36% compared to was in large part down to the perfor- 2013 (an increase of 74 companies mance of companies with over 250 em- compared to the previous year), mak- ployees, whose turnover grew by 17.82%. ing up for the fall of the previous year • Lastly, the contribution towards the and getting back to 2012 levels Spanish GDP of companies active in The figures reveal mixed results in the main • In net terms, 5,034 new jobs have biotechnology (another key indicator key indicators. -

A Acetylcholine Anticholinergic Drugs, 49 Cholinergic Wake-Promoting
Index A C Acetylcholine Cataplexy anticholinergic drugs, 49 GHB, 37 cholinergic wake-promoting system, 48 narcolepsy type 1, 215 cholinesterase inhibitors, 48–49 narcolepsy type 2, 215 milameline, 49 sleep-promoting orexinergic inhibitors, 46 nicotine, 49 SXB, 238–240 receptors, 48 tricyclic antidepressants, 253 sleep-promoting cholinergic Cell replacement therapy, 253 inhibitors, 49 Central sleep apnea (CSA) syndrome, 76 Adenosine Cheyne–Stokes breathing (CSB), 76 adenosine-mediated sleep-promoting Cholinesterase inhibitors system, 41 donepezil, 49 receptors, 42 physostigmine, 48 sleep-promoting adenosine receptor Chronic insomnia agonists, 42 dose discrimination, lack of, 168 wake-promoting adenosine receptor psychiatric comorbidities, 169 antagonists, 42 short-term efficacy and long-term safety, Amantadine, 77 167–168 Armodafinil sleep induction and maintenance, 168–169 chemical structure, 212 Chronic obstructive sleep apnea multiple sclerosis, 222 (OSA), 178–179 narcolepsy, 218 Chronic primary insomnia obstructive sleep apnea, 219–221 adverse effects, 165, 167 pharmacokinetics, 214 comorbid psychiatric disorders, 164 R enantiomer, 213 double-blind trial, 164 safety, 224–225 efficacy, 165 shift work disorder, 223 long-term trial, 165 open-label extension phase, 165 post hoc analysis, 165–166 B psychomotor performance, 164–165 Benzodiazepine Withdrawal Symptom rebound insomnia, 164 Questionnaire (BWSQ), 183 WASO, 164–165 © Springer International Publishing Switzerland 2015 289 A. Guglietta (ed.), Drug Treatment of Sleep Disorders, Milestones -

(12) United States Patent (10) Patent No.: US 9,636,316 B2 Cohen Et Al
USOO9636316B2 (12) United States Patent (10) Patent No.: US 9,636,316 B2 Cohen et al. (45) Date of Patent: May 2, 2017 (54) BACLOFENAND ACAMPROSATE BASED (58) Field of Classification Search THERAPY OF NEUROLOGICAL DISORDERS CPC ... A61K 31/197; A61K 31/445; A61K 31/42: A61K 31/44; A61K 31/195; A61 K (71) Applicant: PHARNEXT, Issy les Moulineaux (FR) 31/185; A61K 31/138: A61K 31/164 (72) Inventors: Daniel Cohen, Saint Cloud (FR); Ilya USPC ................................. 514/567, 555, 568, 665 Chumakov, Vaux-le-Penil (FR); See application file for complete search history. Serguei Nabirochkin, Chatenay-Malabry (FR); Emmanuel Vial, Paris (FR); Mickael Guedj. Paris (56) References Cited (FR) U.S. PATENT DOCUMENTS (73) Assignee: PHARNEXT, Issy les Moulineaux (FR) 6,391922 B1 5/2002 Fogel 8,741,886 B2 6, 2014 Cohen et al. Notice: Subject to any disclaimer, the term of this 2001, 0004640 A1 6/2001 Inada et al. (*) 2001 OO23246 A1 9, 2001 Barritault et al. patent is extended or adjusted under 35 2004.0102525 A1 5, 2004 KOZachuk U.S.C. 154(b) by 0 days. 2008. O18851.0 A1 8, 2008 Yoshino 2009 OO69419 A1 3/2009 Jandeleit et al. (21) Appl. No.: 14/861,169 2009/O197958 A1 8/2009 Sastry et al. 2011 O230659 A1 9/2011 Tsukamoto et al. (22) Filed: Sep. 22, 2015 2012fO27083.6 A1 10, 2012 Cohen et al. (65) Prior Publication Data FOREIGN PATENT DOCUMENTS US 2016/OOOO736A1 Jan. 7, 2016 EP 1 S63 846 8, 2005 EP 1837 O34 9, 2007 WO WO O1, 58.476 8, 2001 WO WO O3,OOT993 1, 2003 Related U.S. -

Tłumaczenie Patentu Europejskiego (19) Pl (11) Pl/Ep 3107898 Polska
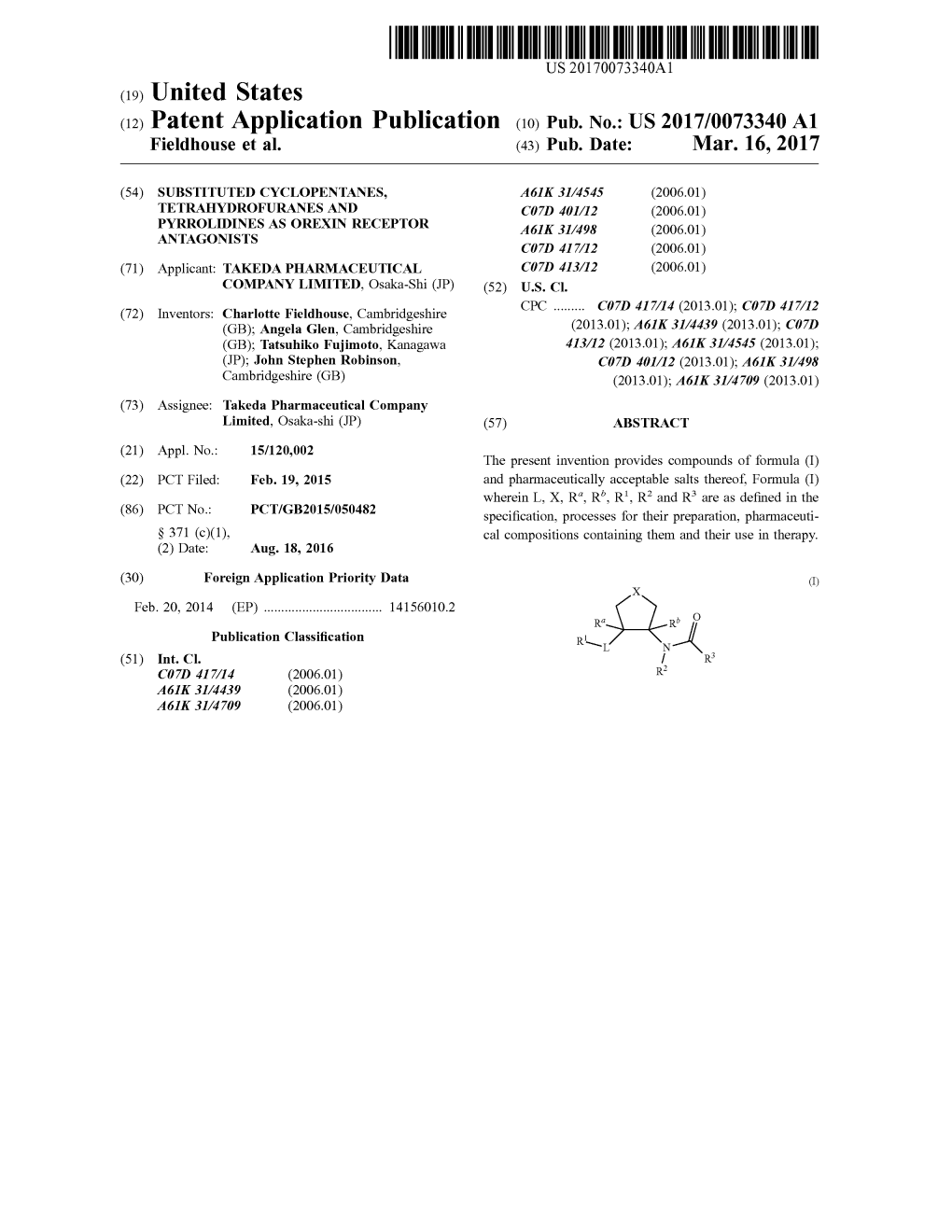
RZECZPOSPOLITA (12) TŁUMACZENIE PATENTU EUROPEJSKIEGO (19) PL (11) PL/EP 3107898 POLSKA (13) T3 (96) Data i numer zgłoszenia patentu europejskiego: (51) Int.Cl. 19.02.2015 15706517.8 C07D 213/74 (2006.01) C07D 239/42 (2006.01) C07D 249/06 (2006.01) (97) O udzieleniu patentu europejskiego ogłoszono: C07D 271/07 (2006.01) 06.01.2021 Europejski Biuletyn Patentowy 2021/01 C07D 231/12 (2006.01) Urząd Patentowy EP 3107898 B1 Rzeczypospolitej A61K 31/44 (2006.01) Polskiej A61K 31/506 (2006.01) A61P 25/18 (2006.01) (54) Tytuł wynalazku: 1,2-PODSTAWIONE CYKLOPENTANY JAKO ANTAGONIŚCI RECEPTORÓW OREKSYN (30) Pierwszeństwo: 20.02.2014 EP 14156011 (43) Zgłoszenie ogłoszono: 28.12.2016 w Europejskim Biuletynie Patentowym nr 2016/52 (45) O złożeniu tłumaczenia patentu ogłoszono: 14.06.2021 Wiadomości Urzędu Patentowego 2021/12 (73) Uprawniony z patentu: Takeda Pharmaceutical Company Limited, Osaka, JP (72) Twórca(y) wynalazku: CHARLOTTE FIELDHOUSE, Cambridge, GB ANGELA GLEN, Cambridge, GB STEPHANIE MAINE, Cambridge, GB T3 TATSUHIKO FUJIMOTO, Fujisawa, JP JOHN STEPHEN ROBINSON, Cambridge, GB (74) Pełnomocnik: rzecz. pat. Tomasz Rychlicki 3107898 ZMP IP SP. Z O.O. ul. Puławska 2 lok. 21, budynek B 02-566 Warszawa PL/EP Uwaga: W ciągu dziewięciu miesięcy od publikacji informacji o udzieleniu patentu europejskiego, każda osoba może wnieść do Europejskiego Urzędu Patentowego sprzeciw dotyczący udzielonego patentu europejskiego. Sprzeciw wnosi się w formie uzasadnionego na piśmie oświadczenia. Uważa się go za wniesiony dopiero z chwilą wniesienia opłaty za sprzeciw (Art. 99 (1) Konwencji o udzielaniu patentów europejskich). 1,2-PODSTAWIONE CYKLOPENTANY JAKO ANTAGONIŚCI RECEPTORÓW OREKSYN Opis Niniejszy wynalazek dotyczy pochodnych amidów, sposobów ich wytwarzania, zawierających je kompozycji farmaceutycznych i ich zastosowania w terapii, zwłaszcza w leczeniu lub zapobieganiu stanom związanym z receptorem oreksyny podtypu 1. -

The Effect of the Short Term Use of Zolpidem MR on Poor Sleep, Daily Pain and Depression in Arthritis Patients
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Wits Institutional Repository on DSPACE The effect of the short term use of Zolpidem MR on poor sleep, daily pain and depression in arthritis patients By Daniela Benjamin Supervisors Dr Alison Bentley Dr Karine Scheuermaier A dissertation submitted to the Faculty of Health Science, University of the Witwatersrand, Johannesburg, in fulfillment of the requirements for the degree of Master of Science in medicine. Johannesburg 2014 i DECLARATION I declare that this dissertation is my own, unaided work. It is being submitted for the degree of Master of Science in medicine in the University of the Witwatersrand, Johannesburg. It has not been submitted before for any degree or examination in any other University. ________________________________ Daniela Benjamin ____ th day of_________October___________2014 ii ABSTRACT Introduction: The presence of pain during sleep causes patients with chronic daily pain, such as in Rheumatoid and Osteoarthritis, to experience insomnia, fragmented sleep and an increased number of night-time awakenings. This poor sleep results in an increased sensitivity to pain during the day. The effect of improving sleep on pain, sleep and mood after taking Zolpidem MR was the aim of this study. Methods: 11 patients from Chris Hani Baragwanath Hospital in Soweto South Africa who reported insomnia and daily pain spent 4 weeks in this crossover design, double blinded, placebo controlled study. Week 1- baseline, week 2 and 4 were Intervention weeks – either placebo or Zolpidem MR, week 3 was a Washout week. Data collected included actigraphy, McGill Pain Questionnaire, PSQI, BDI, physical activity questionnaire and daily sleep and pain diaries containing VAS scales for sleep and pain. -

Syllabi/Slides for This Program Are a Supplement to the Live CME Session and Are Not Intended for Other Purposes
12:50 – 1:50 PM Disclosures This session is supported by an independent educational grant from Eisai Inc. Managing Sleep Maintenance Insomnia in Older Adults The following relationships exist related to this presentation: ► Russell P. Rosenberg, PhD: Advisory Board for Harmony Biosciences, LLC and Jazz Pharmaceuticals, Inc. Consultant for Bose Corporation and Eisai Inc. Contracted Research for SPEAKER Eisai Inc.; Fitbit, Inc.; Idorsia Pharmaceuticals Ltd; Johnson & Johnson; and Merck & Co., Inc. Russell P. Rosenberg, PhD Services Provided for Promotional Purposes (not for CME/CE Services) for Jazz Pharmaceuticals, Inc. Off-Label/Investigational Discussion ► In accordance with pmiCME policy, faculty have been asked to disclose discussion of unlabeled or unapproved use(s) of drugs or devices during the course of their presentations. ASK QUESTIONS USING OUR NEW SOCIAL Q&A FEATURE! Learning Objectives Navigate to www.midwest.cnf.io • Describe current challenges associated with managing sleep maintenance insomnia, especially in older and elderly patients • Utilize currently available and emerging treatments to manage sleep Ask a maintenance insomnia, including those that avoid residual next-day Question effects, especially in older and elderly populations Click a Session Up-Vote a Question Syllabi/slides for this program are a supplement to the live CME session and are not intended for other purposes. Agenda • Defining insomnia • Impact of sleep maintenance insomnia • Sleep maintenance insomnia: • Current management approaches and unmet needs in older adults • Novel and emerging agents • Practical management approaches Pre-Activity Little Difference in Diagnostic Criteria DSM-5 Diagnostic Criteria ICSD-3 Diagnostic Criteria A. A predominant complaint of dissatisfaction with sleep quantity or A. -

Tackling Insomnia As Part of the Complete Approach to Mental Healthcare
Tackling Insomnia as Part of the Complete Approach to Mental Healthcare Karl Doghramji, MD Professor of Psychiatry, Neurology, and Medicine Medical Director, Jefferson Sleep Disorders Center Thomas Jefferson University Philadelphia, Pennsylvania Educational grant support was provided from Eisai. Faculty Disclosure • Dr. Doghramji: Consultant—Eisai, Purdue, Merck, Pfizer; Stock—Merck. Disclosure • The faculty have been informed of their responsibility to disclose to the audience if they will be discussing off-label or investigational use(s) of drugs, products, and/or devices (any use not approved by the US Food and Drug Administration). – Dr. Doghramji will be discussing off-label use of medications in this presentation and will identify those medications. • Applicable CME staff have no relationships to disclose relating to the subject matter of this activity. • This activity has been independently reviewed for balance. Learning Objectives • Discuss the relationship between insomnia and comorbid psychiatric disorders and the resulting implications for diagnosis and co-treatment • Evaluate current guideline recommendations for insomnia management with respect to the present standard of care and unmet needs with existing therapies • Review the latest clinical data surrounding the mechanisms of action and risk/benefit profiles of emerging pharmacotherapies for insomnia for informed therapeutic decision-making Mr. A • 58-year-old accountant, c/o unrefreshing sleep • Onset 4 months ago • Frequency 4 to 5 nights/week • Mind “spins” at bedtime • Feels washed out during day; low energy • Moody, irritable • Curtailed social activities • Medical history: Hypertension, controlled with losartan • Exam: Nl vital signs, BMI 38 • MSE: Psychomotor slowing; mood “fine”. Affect restricted, no h/s ideation, sensorium clear. Cognitive functions intact • Recent blood tests, including CBC, blood chemistry tests, LFTs, and TSH are WNL What additional criterion must be met to satisfy criteria for DSM-5 insomnia disorder? 1. -

Download Product Insert (PDF)
PRODUCT INFORMATION Lorediplon Item No. 23868 F CAS Registry No.: 917393-39-6 N Formal Name: N-[2-fluoro-5-[3-(2-thienylcarbonyl) O pyrazolo[1,5-a]pyrimidin-7-yl]phenyl]- N-methyl-acetamide MF: C H FN O S 20 15 4 2 N FW: 394.4 N Purity: ≥98% N UV/Vis.: λmax: 230, 312, 343 nm S Supplied as: A crystalline solid O Storage: -20°C Stability: ≥2 years Information represents the product specifications. Batch specific analytical results are provided on each certificate of analysis. Laboratory Procedures Lorediplon is supplied as a crystalline solid. A stock solution may be made by dissolving the lorediplon in the solvent of choice. Lorediplon is soluble in organic solvents such as DMSO and dimethyl formamide (DMF), which should be purged with an inert gas. The solubility of lorediplon in these solvents is approximately 14 and 25 mg/ml, respectively. Lorediplon is sparingly soluble in aqueous buffers. For maximum solubility in aqueous buffers, lorediplon should first be dissolved in DMF and then diluted with the aqueous buffer of choice. Lorediplon has a solubility of approximately 0.5 mg/ml in a 1:1 solution of DMF:PBS (pH 7.2) using this method. We do not recommend storing the aqueous solution for more than one day. Description 1 Lorediplon is a ligand for α1 subunit-containing GABAA receptors. In vivo, lorediplon inhibits spontaneous motor activity and increases duration of sleep in mice (ED50s = 0.13 and 1.2 mg/kg, respectively). It selectively inhibits spontaneous motor activity, which is driven by α1 subunit-containing GABAA receptors, 2 over modification of muscular tone in mice, an α2 subunit-containing GABAA receptor-stimulated activity. -

GABA Receptor Gamma-Aminobutyric Acid Receptor; Γ-Aminobutyric Acid Receptor
GABA Receptor Gamma-aminobutyric acid Receptor; γ-Aminobutyric acid Receptor GABA receptors are a class of receptors that respond to the neurotransmitter gamma-aminobutyric acid (GABA), the chief inhibitory neurotransmitter in the vertebrate central nervous system. There are two classes of GABA receptors: GABAA and GABAB. GABAA receptors are ligand-gated ion channels (also known as ionotropic receptors), whereas GABAB receptors are G protein-coupled receptors (also known asmetabotropic receptors). It has long been recognized that the fast response of neurons to GABA that is blocked by bicuculline and picrotoxin is due to direct activation of an anion channel. This channel was subsequently termed the GABAA receptor. Fast-responding GABA receptors are members of family of Cys-loop ligand-gated ion channels. A slow response to GABA is mediated by GABAB receptors, originally defined on the basis of pharmacological properties. www.MedChemExpress.com 1 GABA Receptor Agonists, Antagonists, Inhibitors, Activators & Modulators (+)-Bicuculline (+)-Kavain (d-Bicuculline) Cat. No.: HY-N0219 Cat. No.: HY-B1671 (+)-Bicuculline is a light-sensitive competitive (+)-Kavain, a main kavalactone extracted from Piper antagonist of GABA-A receptor. methysticum, has anticonvulsive properties, attenuating vascular smooth muscle contraction through interactions with voltage-dependent Na+ and Ca2+ channels. Purity: 99.97% Purity: 99.98% Clinical Data: No Development Reported Clinical Data: Launched Size: 10 mM × 1 mL, 50 mg, 100 mg Size: 10 mM × 1 mL, 5 mg, 10 mg (-)-Bicuculline methobromide (-)-Bicuculline methochloride (l-Bicuculline methobromide) Cat. No.: HY-100783 (l-Bicuculline methochloride) Cat. No.: HY-100783A (-)-Bicuculline methobromide (l-Bicuculline (-)-Bicuculline methochloride (l-Bicuculline methobromide) is a potent GABAA receptor methochloride) is a potent GABAA receptor antagonist.