The Role of Excited Oxygen Molecules in the Formation of the Secondary Ozone Layer at 87 to 97 Km
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mission to Jupiter
This book attempts to convey the creativity, Project A History of the Galileo Jupiter: To Mission The Galileo mission to Jupiter explored leadership, and vision that were necessary for the an exciting new frontier, had a major impact mission’s success. It is a book about dedicated people on planetary science, and provided invaluable and their scientific and engineering achievements. lessons for the design of spacecraft. This The Galileo mission faced many significant problems. mission amassed so many scientific firsts and Some of the most brilliant accomplishments and key discoveries that it can truly be called one of “work-arounds” of the Galileo staff occurred the most impressive feats of exploration of the precisely when these challenges arose. Throughout 20th century. In the words of John Casani, the the mission, engineers and scientists found ways to original project manager of the mission, “Galileo keep the spacecraft operational from a distance of was a way of demonstrating . just what U.S. nearly half a billion miles, enabling one of the most technology was capable of doing.” An engineer impressive voyages of scientific discovery. on the Galileo team expressed more personal * * * * * sentiments when she said, “I had never been a Michael Meltzer is an environmental part of something with such great scope . To scientist who has been writing about science know that the whole world was watching and and technology for nearly 30 years. His books hoping with us that this would work. We were and articles have investigated topics that include doing something for all mankind.” designing solar houses, preventing pollution in When Galileo lifted off from Kennedy electroplating shops, catching salmon with sonar and Space Center on 18 October 1989, it began an radar, and developing a sensor for examining Space interplanetary voyage that took it to Venus, to Michael Meltzer Michael Shuttle engines. -

Global Ionosphere-Thermosphere-Mesosphere (ITM) Mapping Across Temporal and Spatial Scales a White Paper for the NRC Decadal
Global Ionosphere-Thermosphere-Mesosphere (ITM) Mapping Across Temporal and Spatial Scales A White Paper for the NRC Decadal Survey of Solar and Space Physics Andrew Stephan, Scott Budzien, Ken Dymond, and Damien Chua NRL Space Science Division Overview In order to fulfill the pressing need for accurate near-Earth space weather forecasts, it is essential that future measurements include both temporal and spatial aspects of the evolution of the ionosphere and thermosphere. A combination of high altitude global images and low Earth orbit altitude profiles from simple, in-the-medium sensors is an optimal scenario for creating continuous, routine space weather maps for both scientific and operational interests. The method presented here adapts the vast knowledge gained using ultraviolet airglow into a suggestion for a next-generation, near-Earth space weather mapping network. Why the Ionosphere, Thermosphere, and Mesosphere? The ionosphere-thermosphere-mesosphere (ITM) region of the terrestrial atmosphere is a complex and dynamic environment influenced by solar radiation, energy transfer, winds, waves, tides, electric and magnetic fields, and plasma processes. Recent measurements showing how coupling to other regions also influences dynamics in the ITM [e.g. Immel et al., 2006; Luhr, et al, 2007; Hagan et al., 2007] has exposed the need for a full, three- dimensional characterization of this region. Yet the true level of complexity in the ITM system remains undiscovered primarily because the fundamental components of this region are undersampled on the temporal and spatial scales that are necessary to expose these details. The solar and space physics research community has been driven over the past decade toward answering scientific questions that have a high level of practical application and relevance. -

Aurorae and Airglow Results of the Research on the International
VARIATIONS IN THE Ha EMISSION AND HYDROGEN DISTRIBUTION IN THE UPPER ATMOSPHERE AND THE GEOCORONA L. M. Fishkova and N. M. Martsvaladze ABSTRACT: The variations in intensity of the hydrogen emission showed a maximum during the minimum of solar activity in 1962-1963. The distribution of hydrogen content at altitudes from 400 to 1300 km was calculated. It in creased during the period of the minimum in the solar cycle. No concentration of H, emis sion around the ecliptic was detected. During the International Quiet Solar Year, spectral observa tions of the intensity of H, emission 6563 a in the airglow spec trum were conducted in Abastumani [l, 21. Continuous observations from f ,hyleighs "w 1953 to 1964 provided for finding the annual variations in H, intensity related to the cycle of solar activ ity (Fig. 1). With a decrease in solar activity, the H, intensity increased and reached a maximum in 1962-1963 while, from 1964, it again began to decrease. Such a variation in the H, intensity can be explained by the variations in Fig. 1. Variations in the the amount of hydrogen during the Average Annual Intensities solar cycle. With a decrease in of H, Emission During the the solar activity, the temperature Cycle of Solar Activity. of the thermopause decreases , which then leads to an increase in the concentration of atomic hydrogen in the exosphere and the geocorona. For example, theoretical calculations [SI show that a change in temperature of the themopause from - 2000 to - 1000° K during the transition from maximum to minimum solar activity can bring about an increase in the hydrogen concentration at altitudes of 500-3000 km, almost by one order of magnitude. -

A Tenuous, Collisional Atmosphere on Callisto
A tenuous, collisional atmosphere on Callisto Shane R. Carberry Mogana;b;c;∗, Orenthal J. Tuckerd, Robert E. Johnsone;f , Audrey Vorburgerg, Andre Gallig, Benoit Marchandh, Angelo Tafunii, Sunil Kumarc, Iskender Sahina, Katepalli R. Sreenivasana;b;f aTandon School of Engineering, New York University, New York, USA; bCenter for Space Sci- ence, New York University Abu Dhabi, Abu Dhabi, UAE; cDivision of Engineering, New York University Abu Dhabi, Abu Dhabi, UAE; dNASA Goddard Space Flight Center, Maryland, USA; eUniversity of Virginia, Virginia, USA; f Physics Department, New York University, New York, USA; gPhysikalisches Institut, University of Bern, Bern, Switzerland; hHigh Per- formance and Research Computing Center, New York University Abu Dhabi, UAE; iSchool of Applied Engineering and Technology, New Jersey Institute of Technology, New Jersey, USA Abstract A simulation tool which utilizes parallel processing is developed to describe molecu- lar kinetics in 2D, single- and multi-component atmospheres on Callisto. This expands on our previous study on the role of collisions in 1D atmospheres on Callisto composed of radiolytic products (Carberry Mogan et al., 2020) by implementing a temperature gradient from noon to midnight across Callisto's surface and introducing sublimated water vapor. We compare single-species, ballistic and collisional O2,H2 and H2O at- mospheres, as well as an O2+H2O atmosphere to 3-species atmospheres which contain H2 in varying amounts. Because the H2O vapor pressure is extremely sensitive to the surface temperatures, the density drops several orders of magnitude with increasing distance from the subsolar point, and the flow transitions from collisional to ballistic accordingly. In an O2+H2O atmosphere the local temperatures are determined by H2O near the subsolar point and transition with increasing distance from the subsolar point to being determined by O2. -

Temporal Variability of Atomic Hydrogen from the Mesopause To
PUBLICATIONS Journal of Geophysical Research: Space Physics RESEARCH ARTICLE Temporal Variability of Atomic Hydrogen From 10.1002/2017JA024998 the Mesopause to the Upper Thermosphere Key Points: Liying Qian1 , Alan G. Burns1 , Stan S. Solomon1 , Anne K. Smith2 , Joseph M. McInerney1 , • In the mesopause, hydrogen density is 3 1,2 1 3 1,2 higher in summer than in winter, and Linda A. Hunt , Daniel R. Marsh , Hanli Liu ,MartinG.Mlynczak , and Francis M. Vitt higher at solar minimum than at 1 2 solar maximum High Altitude Observatory, National Center for Atmospheric Research, Boulder, CO, USA, Atmospheric Chemistry 3 • MSIS exhibits reverse seasonal Observations and Modeling Laboratory, National Center for Atmospheric Research, Boulder, CO, USA, NASA Langley variation than WACCM and SABER Research Center, Hampton, VA, USA do in the mesopause region • In the upper thermosphere, H is higher at solar minimum than solar Abstract We investigate atomic hydrogen (H) variability from the mesopause to the upper thermosphere, maximum, higher in winter than summer, and also higher at night on time scales of solar cycle, seasonal, and diurnal, using measurements made by the Sounding of the than day Atmosphere using Broadband Emission Radiometry (SABER) instrument on the Thermosphere Ionosphere Mesosphere Energetics Dynamics satellite, and simulations by the National Center for Atmospheric Research Whole Atmosphere Community Climate Model-eXtended (WACCM-X). In the mesopause region (85 to Correspondence to: 95 km), the seasonal and solar cycle variations of H simulated by WACCM-X are consistent with those from L. Qian, SABER observations: H density is higher in summer than in winter, and slightly higher at solar minimum than [email protected] at solar maximum. -

Cubesat-Based Science Missions for Geospace and Atmospheric Research
National Aeronautics and Space Administration NATIONAL SCIENCE FOUNDATION (NSF) CUBESAT-BASED SCIENCE MISSIONS FOR GEOSPACE AND ATMOSPHERIC RESEARCH annual report October 2013 www.nasa.gov www.nsf.gov LETTERS OF SUPPORT 3 CONTACTS 5 NSF PROGRAM OBJECTIVES 6 GSFC WFF OBJECTIVES 8 2013 AND PRIOR PROJECTS 11 Radio Aurora Explorer (RAX) 12 Project Description 12 Scientific Accomplishments 14 Technology 14 Education 14 Publications 15 Contents Colorado Student Space Weather Experiment (CSSWE) 17 Project Description 17 Scientific Accomplishments 17 Technology 18 Education 18 Publications 19 Data Archive 19 Dynamic Ionosphere CubeSat Experiment (DICE) 20 Project Description 20 Scientific Accomplishments 20 Technology 21 Education 22 Publications 23 Data Archive 24 Firefly and FireStation 26 Project Description 26 Scientific Accomplishments 26 Technology 27 Education 28 Student Profiles 30 Publications 31 Cubesat for Ions, Neutrals, Electrons and MAgnetic fields (CINEMA) 32 Project Description 32 Scientific Accomplishments 32 Technology 33 Education 33 Focused Investigations of Relativistic Electron Burst, Intensity, Range, and Dynamics (FIREBIRD) 34 Project Description 34 Scientific Accomplishments 34 Education 34 2014 PROJECTS 35 Oxygen Photometry of the Atmospheric Limb (OPAL) 36 Project Description 36 Planned Scientific Accomplishments 36 Planned Technology 36 Planned Education 37 (NSF) CUBESAT-BASED SCIENCE MISSIONS FOR GEOSPACE AND ATMOSPHERIC RESEARCH [ 1 QB50/QBUS 38 Project Description 38 Planned Scientific Accomplishments 38 Planned -

The Exosphere, Leading to a Much More Descriptive Science
UNCLASSIFIED 1070 SSPPAACCEE EENNVVIIIRROONNMMEENNTT TTEECCHHNNOOLLOO GGIIIEESS GEOCORONA IN LYMAN Contract F19628 Contract Justin Technologies public distribution Notice: This document is released for May 2012 EXOSPHERIC HYDROGEN EXOSPHERIC J. J. OBSERVATIONS OF THE OF OBSERVATIONS Bailey ATOM DISTRIBUTIONS ATOM . THREE - 03 by by - C Space Space Environment - OBTAINED FROM OBTAINED 0076 SET TR 2012 - DIMENSIONAL - - 001 ALPHA THREE-DIMENSIONAL EXOSPHERIC HYDROGEN ATOM DISTRIBUTIONS OBTAINED FROM OBSERVATIONS OF THE GEOCORONA IN LYMAN-ALPHA by Justin J. Bailey A Dissertation Presented to the FACULTY OF THE USC GRADUATE SCHOOL UNIVERSITY OF SOUTHERN CALIFORNIA In Partial Fulfillment of the Requirements for the Degree DOCTOR OF PHILOSOPHY (ASTRONAUTICAL ENGINEERING) May 2012 Copyright 2012 Justin J. Bailey for my wife, Natalie, a bona fide yogini who at times had to wrap her hands around my head to keep my brain from falling out ii Acknowledgements My opportunity to contribute in this field was established and supported by many different people. After finishing with an M.S. in Astronautical Engineering, I walked into Professor Mike Gruntman’s office hoping to learn more about space physics and aeronomy. He proceeded to draw the geocorona in assorted colors on a whiteboard, and it was then that the content of this dissertation was born. Of course, none of this research would have been possible were it not for his expertise and guidance. I would like to thank my advisory committee (Professors Daniel Erwin, Joseph Kunc, W. Kent Tobiska, and Darrell Judge) for their many insightful observations. I am especially indebted to Dr. Tobiska for numerous opportunities to learn more about the solar output and how it drives the exosphere, leading to a much more descriptive science discussion in the relevant sections. -

Soft X-Ray Emissions from Planets, Moons, and Comets
SOFT X-RAY EMISSIONS FROM PLANETS, MOONS, AND COMETS A. Bhardwaj(1), G. R. Gladstone(2), R. F. Elsner(3), J. H. Waite, Jr.(4), D. Grodent(5), T. E. Cravens(6), R. R. Howell(7), A. E. Metzger(8), N. Ostgaard(9), A. N. Maurellis(10), R. E. Johnson(11), M. C. Weisskopf(3), T. Majeed(4), P. G. Ford(12), A. F. Tennant(3), J. T. Clarke(13), W. S. Lewis(2), K. C. Hurley(9), F. J. Crary(4), E. D. Feigelson(14), G. P. Garmire(14), D. T. Young(4), M. K. Dougherty(15), S. A. Espinosa(16), J.-M. Jahn(2) (1)Space Physics Laboratory, Vikram Sarabhai Space Centre, Trivandrum 695022, India, [email protected] (2)Southwest Research Institute, San Antonio, TX 78228, USA (3)NASA Marshall Space Flight Center, Huntsville, AL 35812, USA (4)University of Michigan, Ann Arbor, MI 48109, USA (5)LPAP, University of Liege, Belgium (6)University of Kansas, Lawrence, KS 66045, USA (7)University of Wyoming, Laramie, WY 82071, USA (8)Jet Propulsion Laboratory, Pasadena, CA 91109, USA (9)University of California at Berkeley, Berkeley, CA 94720, USA (10)SRON National Institute for Space Research, Utrecht, The Netherlands (11)University of Virginia, Charlottesville, VA 22903, USA (12)Massachusetts Institute of Technology, Cambridge, MA 02139, USA (13)Boston University, Boston, MA 02215, USA (14)Pennsylvania State University, State College, PA 16802, USA (15)Blackett Laboratory, Imperial College, London, England (16)Max-Planck-Institut für Aeronomie, Katlenburg-Lindau, Germany ABSTRACT altitude balloon-, rocket-, and satellite-based instruments. But to observe most of the soft x-ray band one has to be A wide variety of solar system bodies are now known to above ~100 km from Earth's surface. -

Detection of a Hydrogen Corona at Callisto in Hst/Stis Lyman-Α Images
Lunar and Planetary Science XLVIII (2017) 1861.pdf DETECTION OF A HYDROGEN CORONA AT CALLISTO IN HST/STIS LYMAN-α IMAGES. J. Alday1, L. Roth1, N. Ivchenko1, T. Becker2, and K. D. Retherford2 1 KTH Royal Institute of Technology, School of Electrical Engineering, Stockholm, Sweden. 2 Southwest Research Institute, San Antonio, TX, USA. Introduction: In December 2001, far-ultraviolet reflected sunlight to regions above the limb, we devel- spectral images of Jupiter's moon Callisto were ob- op a forward model that takes into account all three tained by the Space Telescope Imaging Spectrograph contributions to the Lyman-α signal as well as the PSF (STIS) of the Hubble Space Telescope (HST) in the effects. Simultaneously fitting parameters for Callis- search for carbon and oxygen atmospheric emissions. to’s surface reflectance (or albedo) and the H abun- On two occasions, the leading and trailing hemispheres dance, we derive constraints for the corona. were observed with the moon at eastern and western Results: We find that faint atmospheric Lyman-α elongations, respectively. No measurable oxygen or emissions extending up to several moon radii away are carbon emissions were detected [1]. We re-analyzed present in addition to the solar Lyman-α flux reflected the hydrogen Lyman-α (1216 Å) images in these 2001 off the surface. We show that the detected atmospheric STIS observations in the search for emissions from Lyman-α emissions are consistent with an escaping hydrogen-bearing atmospheric constituents. hydrogen exosphere with a vertical column density in Observations and Method: During two visits on the range of (3 – 9) × 1011 H/cm2. -

ESA Space Weather STUDY Alcatel Consortium
ESA Space Weather STUDY Alcatel Consortium SPACE Weather Parameters WP 2100 Version V2.2 1 Aout 2001 C. Lathuillere, J. Lilensten, M. Menvielle With the contributions of T. Amari, A. Aylward, D. Boscher, P. Cargill and S.M. Radicella 1 2 1 INTRODUCTION........................................................................................................................................ 5 2 THE MODELS............................................................................................................................................. 6 2.1 THE SUN 6 2.1.1 Reconstruction and study of the active region static structures 7 2.1.2 Evolution of the magnetic configurations 9 2.2 THE INTERPLANETARY MEDIUM 11 2.3 THE MAGNETOSPHERE 13 2.3.1 Global magnetosphere modelling 14 2.3.2 Specific models 16 2.4 THE IONOSPHERE-THERMOSPHERE SYSTEM 20 2.4.1 Empirical and semi-empirical Models 21 2.4.2 Physics-based models 23 2.4.3 Ionospheric profilers 23 2.4.4 Convection electric field and auroral precipitation models 25 2.4.5 EUV/UV models for aeronomy 26 2.5 METEOROIDS AND SPACE DEBRIS 27 2.5.1 Space debris models 27 2.5.2 Meteoroids models 29 3 THE PARAMETERS ................................................................................................................................ 31 3.1 THE SUN 35 3.2 THE INTERPLANETARY MEDIUM 35 3.3 THE MAGNETOSPHERE 35 3.3.1 The radiation belts 36 3.4 THE IONOSPHERE-THERMOSPHERE SYSTEM 36 4 THE OBSERVATIONS ........................................................................................................................... -

Auroral Zone Effects on Hydrogen Geocorona Structure and Variability
Planet. Space Sci., Vol. 33, No. 5, pp. 499-505, 1985 0032-0633/85 $3.00 + O.MI Printed in Great Britain. 0 1985 Pergamon Press Ltd. AURORAL ZONE EFFECTS ON HYDROGEN GEOCORONA STRUCTURE AND VARIABILITY T. E. MOORE, A. P. BIDDLE and J. H. WAITE, Jr. Space Science Laboratory, NASA/Marshall Space Flight Center, Huntsville, AL 35812, U.S.A. and T. L. KILLEEN University of Michigan, Ann Arbor, Michigan, U.S.A. (Received 16 Octobera&984) Abstract-The instantaneous structure of planetary exospheres is determined by the time history of energy dissipation, chemical, and transport processes operative during a prior time interval set by intrinsic atmospheric time scales. The complex combination of diurnal and magnetospheric activity modulations imposed on the Earth’s upper atmosphere no doubt produce an equally complex response, especially in hydrogen, which escapes continuously at exospheric temperatures. Vidal-Madjar and Thomas (1978) have discussed some of the persistent large scale structure which is evident in satellite ultraviolet observations of hydrogen, noting in particular a depletion at high latitudes which is further discussed by Thomas and Vidal- Madjar (1978). The latter authors discussed various causes of the H density depletion, including local neutral temperature enhancements and enhanced escape rates due to polar wind H + plasma flow or high latitude ion heating followed by charge exchange. We have reexamined the enhancement of neutral escape by plasma effects including the recently observed phenomenon of low altitude transverse ion acceleration. We find that, while significant fluxes ofneutral H should be produced by this phenomenon in the aurora1 zone, this process is probably insufficient to account for the observed polar depletion. -
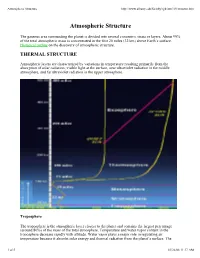
Atmospheric Structure
Atmospheric Structure http://www.albany.edu/faculty/rgk/atm101/structur.htm Atmospheric Structure The gaseous area surrounding the planet is divided into several concentric strata or layers. About 99% of the total atmospheric mass is concentrated in the first 20 miles (32 km) above Earth's surface. Historical outline on the discovery of atmospheric structure. THERMAL STRUCTURE Atmospheric layers are characterized by variations in temperature resulting primarily from the absorption of solar radiation; visible light at the surface, near ultraviolet radiation in the middle atmosphere, and far ultraviolet radiation in the upper atmosphere. Troposphere The troposphere is the atmospheric layer closest to the planet and contains the largest percentage (around 80%) of the mass of the total atmosphere. Temperature and water vapor content in the troposphere decrease rapidly with altitude. Water vapor plays a major role in regulating air temperature because it absorbs solar energy and thermal radiation from the planet's surface. The 1 of 5 01/26/08 11:17 AM Atmospheric Structure http://www.albany.edu/faculty/rgk/atm101/structur.htm troposphere contains 99 % of the water vapor in the atmosphere. Water vapor concentrations vary with latitude. They are greatest above the tropics, where they may be as high as 3 %, and decrease toward the polar regions. All weather phenomena occur within the troposphere, although turbulence may extend into the lower portion of the stratosphere. Troposphere means "region of mixing" and is so named because of vigorous convective air currents within the layer. The upper boundary of the layer, known as the tropopause, ranges in height from 5 miles (8 km) near the poles up to 11 miles (18 km) above the equator.