Forensic Biology Unit Quality Assurance Manual FBQA01
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Forensic Biology 205 Administration Building • 419-372-2015
Fall 2020 Bachelor of Science in Forensic Science Specialization in Forensic Biology 205 Administration Building • 419-372-2015 BG Perspective (BGP) Requirements FSCI Major Core Requirements (38 Hrs.) Must complete at least 1 course in each of the following: Hrs Grade English Composition and Oral Communication 4 BIOL 2040 Concepts in Biology I Course Credits 4 BIOL 2050 Concepts in Biology II 4 BIOL 3310 Human Anatomy & Physiology Quantitative Literacy 4 BIOL 3320 Human Anatomy & Physiology II _____________________________ ________ 3 CHEM 1770 Intro to Forensic Science 3 CRJU 4400 Law, Evidence, & Procedures in Must Complete at least 2 courses in each of the following: Forensic Science Humanities and the Arts 3 CRJU 4510 Criminal Justice Ethics 3 MATH 2470 Fund. of Statistics 5 PHYS 2010 or 2110 University Physics I 5 PHYS 2020 or 2120 University Physics II Natural Sciences - at least one Lab Science required FSCI Forensic Biology Specialization Requirements (16 hrs.) Social and Behavioral Sciences 4 BIOL 3500 Genetics 3 BIOL 4080 Molecular Biology 3 BIOL 4230 OR FSCI 4230 Forensic Biology Complete total required BGP credit hours by selecting courses from any 3 BIOL 4240 OR FSCI 4240 Forensic DNA Analysis of the above categories: 3 FSCI 4890 Internship OR FSCI 4990 Capstone Additional Requirements (25-26 Hrs.) These courses also fulfill the requirements for a minor in chemistry. Consult with an advisor about declaring the minor. University Requirements Designated courses in Humanities and the Arts and the Social and Behavorial Sciences -

Syllabus - Forensic Biology Section-C
SYLLABUS - FORENSIC BIOLOGY SECTION-C UNIT-I Forensic Biology: Definition and scope Importance, nature, location, collection and preservation of biological exhibits and crime scene investigation of biological evidence.Cell: Definition, Theories, Classification and Significance of Cells in Forensic Science. Cell Organelles and their Functions, Difference between Eukaryotic and Prokaryotic Cell, Difference between Plant and Animal Cell. Cell Division: Definition, Types, Difference between Somatic and Germinal Cell and Totipotency and Apoptosis. Basic Concept in Brief for Anatomy and Physiology of Digestive, Respiratory, Circulatory, Skeleton, Nervous, Excretory and Reproductive System etc. Unit-2 Basic Plant biology- plant cell structure and function, basic plant tissues, modes of plant reproduction, plant classification schemes, subspecialties of forensic botany such as plant morphology, plant anatomy, plant ecology, limnology etc. Essential parts of plants.Plant as evidence.Common poisonous plant and types of plant toxins. Unit-3 Botanical evidences:- Forensic importance, Introduction, types, location, collection, preservation and evaluation of – Wood: types of wood and anatomy, methods of identification and comparison. Leaves: Identification of various types of leaves and their anatomy, methods of comparison.Pollens: Structure, function, methods of identification and comparison. Seeds and spores: structure and formation in fungi, gymnosperm and angiosperm. Forensic Diatomology: Diatoms: Nature, classification, location, structure, life -

Forensic Science 1
Forensic Science 1 catalog. The individual major/degree program listings of classes ensures FORENSIC SCIENCE that a student will meet the minimum curriculum requirements of the College. Description World Languages/Language Requirement Website: http://forensic.unl.edu/ Two units of a world language are required. This requirement is usually Forensic science includes any science that is conducted for use in met with two years of high school language. the legal system. The need for science in the courtroom has greatly increased as a result of legal rulings and the positioning of forensic Minimum Hours Required for Graduation science in popular culture. The forensic science degree program provides The College grants the bachelors degree in programs associated with students with an education in the use of science, mathematics, and agricultural sciences, natural resources, and related programs. Students statistics in legal proceedings. There are four options of study: working toward a degree must earn at least 120 semester hours of credit. A minimum cumulative grade point average of C (2.0 on a 4.0 scale) • Forensic Biology Option must be maintained throughout the course of studies and is required for • Crime Scene Investigation Option graduation. Some degree programs have a higher cumulative grade point • Forensic Chemistry Option average required for graduation. Please check the degree program on its graduation cumulative grade point average. • Pre-Law Option 3-3 Program Grade Rules College Requirements Removal of C-, D, and F Grades College Admission Only the most recent letter grade received in a given course will be used Requirements for admission into the College of Agricultural Sciences in computing a student’s cumulative grade point average if the student and Natural Resources (CASNR) are consistent with general University has completed the course more than once and previously received a admission requirements (one unit equals one high school year): 4 units grade or grades below C in that course. -

Associate of Science to B.S. in Forensic Biology
Guilford College Bi-Lateral Transfer Agreement Proposal Guilford Technical Community College ASSOCIATE OF SCIENCE TO B.S. IN FORENSIC BIOLOGY GENERAL EDUCATION REQUIREMENTS FOUNDATIONS ENGL 102 ENG 111 = ENGL 102 (3T) Historical Perspectives ENG 112 = ENGL 150 + HIST 131 = HIST 103 (6T) Foreign Language SPA 111 = SPAN 101 (3T) Quantitative Literacy MAT 152 = MATH 112 (4T) EXPLORATIONS – BREADTH Arts MUS 110 = MUS 111 (3T) Business and Policy Studies BUS 110= BUS 120 (3T) Humanities ENG 232 = ENGL 226 (3T) Natural Science BIO 112 = BIOL 112 (4T) Social Science ECO 251=ECON 222 (3T) EXPLORATIONS – CRITICAL PERSPECTIVES Intercultural REL 110 = REL 150 (3T) Social Justice/Environmental Responsibility PHI 240= PHIL 111 (3T) Diversity in the U.S. ENG 232 = ENGL 226 INTERDISCIPLINARY CAPSTONE COURSE IDS 400-level One approved Guilford course MAJOR: FORENSIC BIOLOGY BIO 111 = BIOL 111 Int. Biol.: Molecules & Cells (4T) BIOL 245 Introduction to Forensic Science BIOL 246 Forensic Chemistry BIOL 313 Cell Biology BIOL 341 Human Anatomy and Physiology I Two courses from: BIOL 115 General Botany BIOL 342 Human Anatomy and Physiology II BIOL 434 Biochemistry BIOL 443 Genetics CHM 151=CHEM 111 Chemical Principles I (4T) CHM 152=CHEM 112 Chemical Principles II (4T) PHYS 211 College Physics I PHYS 212 College Physics II One course from MATH 115 Elementary Functions MATH 271=MATH 121 (4T) MATH 122 Calculus II MATH 123 Accelerated Calculus ELECTIVES: MAT 172 = MATH 150 (4T) PED 110= SPST 109 (2T) ACA 122= GST 150 (1T) One Guilford elective at 4 credit hours One Guilford elective at 4 credit hours One Guilford elective at 4 credit hours One Guilford elective at 3 credit hours Students must satisfy all General Education, major, and minor requirements and complete a minimum of 128 credits with at least a “C” (2.0) average in order to earn a baccalaureate degree. -

Ebook Download Forensic Chemistry
FORENSIC CHEMISTRY PDF, EPUB, EBOOK David E. Newton | 208 pages | 15 Nov 2008 | Facts on File Inc | 9780816078004 | English | New York, United States Department of Chemistry and Biochemistry - B.S. Forensic Chemistry Degree Mass Spectrometry MS breaks samples apart and separates the ionized fragments by mass and charge. Generally, forensic chemists are trained in organic chemistry. This ensures that the forensic chemists can run analysis on blood and other body samples to identify DNA. They are also trained in organic chemistry so that they can run toxicology screenings. It is also important for a forensic chemist to have knowledge of physics. There are also forensic chemists who specialize in certain areas, such as chemicals that are tied to explosives or arson. These chemists will be called to a crime scene to look at fire patterns when determining if arson was involved in a fire or they will be called to investigate chemicals associated with a bomb. Once becoming a forensic chemist, there are many places where a forensic chemist could work. A forensic chemist might work for a private lab, or at a national agency like the FBI. Twitter Facebook Instagram Youtube. Back to Crime Library. A mysterious white powder, a blood smear, and a moldy ham sandwich—completely unrelated items to most. But they could be meaningful for forensic chemists, who analyze physical evidence and samples for clues to solve crimes. Television shows such as Bones, CSI, and Dexter have glamorized forensic scientists and made the field more popular, so competition can be intense. However, if you have a strong desire to shape the world of justice by using science to solve crime puzzles, then a career in forensic science could be worth pursuing. -

Forensic Analysis of Expirated Blood
Forensic analysis of expirated blood ANDREA DONALDSON A Thesis submitted for the degree of Master of Science in Biochemistry at the University of Otago, Dunedin, New Zealand January 2010 For my nephew Brayden, who taught me how precious life is and to always follow your heart. ii Abstract In forensic investigations the distinction between expirated bloodstains (blood from the mouth, nose or lungs) and impact spatter (blood from gunshots, explosives, blunt force trauma and/or machinery accidents) is often important but difficult to determine due to their high degree of size similarity, which may result in the patterns being incorrectly classified. Expirated bloodstains on an accused person’s clothing occur when assisting an injured person, a finding which would tend to exonerate that individual. Impact spatter stains on clothing tend to occur due to the proximity of the person to the bloodshedding event, implying guilt. Therefore this project determined the characteristics inherent in each bloodstain type by using high speed digital video analysis and developed a test using PCR analysis to distinguish between the two types of bloodstain patterns to allow for proper bloodstain classification. The current study developed a test involving PCR analysis using DNA from human- specific oral microbes as a biomarker for the presence of saliva and hence oral expirated bloodstains. This PCR method is very specific to human oral Streptococci, with no PCR product being made from human DNA or DNA from soil or other microbes that were tested. It is also very sensitive, detecting as little as 60 fg of target DNA. The PCR was not inhibited by the presence of blood and could detect target DNA in expirated blood for at least 92 days after deposit on cardboard or cotton fabric. -

Informative Textbooks on Forensic DNA (16) the Following Informative Textbooks Are Listed by Publication Date in Ascending Order
Informative Literature for Forensic Biology and DNA prepared by OSAC with valuable additional contributions and organizational recommendations from Dr. John M. Butler and the SWGDAM 2020 Training Guidelines) DRAFT 10/20/2020 Informative Textbooks on Forensic DNA (16) The following informative textbooks are listed by publication date in ascending order with the most recent ones listed last. This list is not comprehensive (e.g., earlier editions of some of these textbooks not included). 1. National Research Council (1996) The Evaluation of Forensic DNA Evidence. National Academy Press: Washington, D.C. 2. Evett, I.W. and Weir, B.S. (1998) Interpreting DNA Evidence: Statistical Genetics for Forensic Scientists. Sinauer Associates: Sunderland MA. 3. Inman, K. and Rudin, N. (2001) Principles and Practice of Criminalistics: The Profession of Forensic Science. CRC Press: Boca Raton. 4. Fung, W.K. and Hu, Y.-Q. (2008) Statistical DNA Forensics: Theory, Methods and Computation. Wiley: Chichester, UK. 5. Butler, J.M. (2010) Fundamentals of Forensic DNA Typing. Elsevier Academic Press: San Diego. 6. Goodwin, W., Linacre, A., Hadi, S. (2011) An Introduction to Forensic Genetics Second Edition. Wiley: Chichester, UK. 7. Butler, J.M. (2012) Advanced Topics in Forensic DNA Typing: Methodology. Elsevier Academic Press: San Diego. 8. Gill, P. (2014) Misleading DNA Evidence: Reasons for Miscarriages of Justice. Elsevier Academic Press: San Diego. 9. Butler, J.M. (2015) Advanced Topics in Forensic DNA Typing: Interpretation. Elsevier Academic Press: San Diego. 10. Balding, D. J. and Steele, C. D. (2015). Weight-of-evidence for Forensic DNA Profiles Second Edition. Wiley: Chichester, UK. 11. Buckleton, J.S., Bright, J.-A., Taylor, D. -

73Rd Aafs Annual Scientific Meeting
AMERICAN ACADEMY OF FORENSIC SCIENCES 73RD AAFS ANNUAL SCIENTIFIC MEETING PROGRAM • FEBRUARY 2021 WELCOME MESSAGE Welcome to the 73rd American Academy of Forensic Sciences Annual Scientific Meeting. We are 100% virtual this year and it is my pleasure and excitement to greet each and every one of you. While we could not be in Houston, we have planned a jammed packed week of science, collegial interactions, collaborations, learning, social networking, and just plain fun! The Academy has nearly 6,500 members representing 70 countries. We usually meet annually in the U.S., but this year we are meeting virtually and worldwide for the first time. I cannot wait to see how many locations across the globe will be represented by our attendees. Jeri D. Ropero-Miller, PhD 2020-21 AAFS President Our meeting theme this year, One Academy Pursuing Justice through Truth and Evidence, has fueled the culture of the Academy since its inception. It has also encouraged more than 1,000 presentations for this week with over 560 oral, more that 380 posters, and countless presenters in the 18 workshops, and special sessions. The Exhibit Hall will be accessible to attendees the entire week. Don’t miss out on all the latest products and technology available to the forensic science industry. You also will want to participate in the Gamification activity by going on a virtual scavenger hunt to earn points towards winning some fun prizes. Don’t forget to visit the online AAFS store between sessions for your chance to purchase meeting apparel. I cannot thank everyone enough for all the effort, dedication, and support for making this virtual event memorable for all. -

NIH Fellowship Training Program- Forensic Science Career Seminar Questions VA Department of Forensic Science Responses from Phds
NIH Fellowship Training Program- Forensic Science Career Seminar Questions VA Department of Forensic Science Responses from PhDs General requirements for employment with VA Department of Forensic Science (DFS): • Must pass criminal background check • Must submit DNA sample • Must be eligible to work in the USA without DFS sponsorship Only 10% of forensic scientific staff have PhDs. Most are in Toxicology section where a PhD is required to testify to pharmacological effects of drugs and alcohol, followed by a several PhDs working in the research and/or casework areas of Forensic Biology, two in Controlled Substances, two in Division of Technical Services. Responses from Virginia Department of Forensic Science PhDs, six Toxicologists, one Drug Chemist, three in Forensic Biology: a) What was your PhD/MS degree in? • Molecular Biology with a Minor in Genetics • Chemistry, specialty Analytical • Biomedical Science • Chemistry (bioanalytical) with MS in Forensic Science • PhD in chemistry (analytical/environmental) • MS in forensic science, PhD in toxicology • Ph.D.in Pharmacology/Toxicology, MS in Forensic Science • Chemistry/Biophysical Chemistry • MFS and PhD in For Tox • Microbiology/Immunology b) What lead you into your current career? • I was looking for something interesting that used my education when I saw an advertisement for a position • Interested in mystery novels, puzzles, brain teasers. Piqued by WVU’s FS program. Did internship with WVState Police in LX or CSI but was erroneously put in Tox which was a fortuitous mistake • Became interested in forensic biology while in grad school. Many of classes and techniques required for degree applied directly to field and offered a viable alternative to academic or industrial research December 14, 2010 1 • High School internship with local police department crime lab doing crime scene and lab work. -

Education and Training in Forensic Science: a Guide for Forensic Science Laboratories, Educational Institutions, and Students U.S
U.S. Department of Justice Office of Justice Programs JUNE 04 National Institute of Justice Special REPORT Education and Training in Forensic Science: A Guide for Forensic Science Laboratories, Educational Institutions, and Students U.S. Department of Justice Office of Justice Programs 810 Seventh Street N.W. Washington, DC 20531 John Ashcroft Attorney General Deborah J. Daniels Assistant Attorney General Sarah V. Hart Director, National Institute of Justice This and other publications and products of the National Institute of Justice can be found at: National Institute of Justice www.ojp.usdoj.gov/nij Office of Justice Programs Partnerships for Safer Communities www.ojp.usdoj.gov JUNE 04 Education and Training in Forensic Science A Guide for Forensic Science Laboratories, Educational Institutions, and Students Developed and Approved by the Technical Working Group for Education and Training in Forensic Science NCJ 203099 Sarah V. Hart Director Findings and conclusions of the research reported here are those of the authors and do not reflect the official position or policies of the U.S. Department of Justice. The National Institute of Justice is a component of the Office of Justice Programs, which also includes the Bureau of Justice Assistance, the Bureau of Justice Statistics, the Office of Juvenile Justice and Delinquency Prevention, and the Office for Victims of Crime. Message From the Director Forensic scientists play a pivotal role in the This publication is especially timely as criminal justice system, providing crucial President Bush has directed DOJ to information about the evidence to the trier undertake a $1 billion, 5-year program to of fact. -

Bloodstain Pattern Analysis Activity Guide
BLOODSTAIN PATTERN ANALYSIS DESCRIPTION OF SESSION This session provides participants with an understanding of bloodstain pattern analysis and how the study of the size, shape, and location of bloodstains can be used to determine the physical events that gave rise to their origin. CATEGORIES . Exploring: Law Enforcement . Exploring: Science . U.S. Department of Education: Law, Public Safety, Corrections & Security . U.S. Department of Education: Government & Public Administration OBJECTIVES By the end of this session, participants will be able to: . List the components of human blood. Identify the mechanisms by which blood spatter patterns are created. Describe how the surface texture of an object affects the shape of individual bloodstains. Describe the directly proportional relationship between stain diameter and dropping height. Create and interpret transfer patterns. Measure the length and width of bloodstains. Calculate the impact angle of bloodstains. SUPPLIES . (1) computer with internet access For each participant: . (1) eye dropper or pipette . (1) bottle of stage blood or red tempura paint . (1) package of white 8½-by-11-inch card stock . (1) meter stick . (1) metric ruler . (1) cake pan or pie plate . Household objects (fork, knife, sponge, hammer, wrench, screwdriver, etc.) . (1) scientific calculator . Impact Angle Determination Practice activity sheet (PDF) . Impact Angle Determination Practice answer key (PDF) PREPARATION See Activity 6 for suggestions of speakers who could attend the meeting or places where participants could visit, and make arrangements as needed. WEBSITES . “Bloodstain Tutorial” (J. Slemko Forensic Consulting): www.bloodspatter.com/bloodstain-tutorial—Overview of bloodstain pattern analysis. “A Simplified Guide to Bloodstain Pattern Analysis” (National Forensic Science Technology Center): www.forensicsciencesimplified.org/blood/how.html—Overview of bloodstain pattern analysis. -
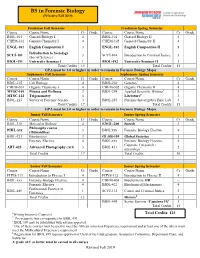
BS in Forensic Biology (Effective Fall 2019)
BS in Forensic Biology (Effective Fall 2019) Freshman Fall Semester Freshman Spring Semester Course Course Name Cr Grade Course Course Name Cr Grade BIOL-101 General Biology I 4 BIOL-102 General Biology II 4 CHEM-101 General Chemistry I 4 CHEM-102 General Chemistry II 4 ENGL-101 English Composition I 3 ENGL-102 English Composition II 3 Introduction to Sociology SCCJ-101 3 SCCJ-104 Introduction to Criminal Justice 3 (Social Science) BIOL-191 University Seminar I 1 BIOL-192 University Seminar II 1 Total Credits 15 Total Credits 15 GPA must be 3.0 or higher in order to remain in Forensic Biology Major Sophomore Fall Semester Sophomore Spring Semester Course Course Name Cr Grade Course Course Name Cr Grade BIOL-215 Cell Biology 4 BIOL-210 Genetics* 4 CHEM-301 Organic Chemistry I 4 CHEM-302 Organic Chemistry II 4 MVSC-101 Fitness and Wellness 2 BIOL-299 Applied Scientific Writing+ 1 MTSC-122 Trigonometry 4 Literature# 3 BIOL-225 Survey of Forensic Science 3 BIOL-255 Forensic/Investigative Biol. Lab 3 Total Credits 17 Total Credits 15 GPA must be 3.0 or higher in order to remain in Forensic Biology Major Junior Fall Semester Junior Spring Semester Course Course Name Cr Grade Course Course Name Cr Grade BIOL-310 Molecular Biology* 4 ENGL-200 Speech 3 Philosophy course PHIL-xxx 3 BIOL xxx Forensic Biology Elective 4 (Humanities) BIOL-321 Biostatistics 3 GLOB-395 Global Societies 3 Forensic Elective 4 BIOL-xxx Forensic Biology Elective 4 Capstone I (research / ART-425 Advanced Photography (Art) 3 BIOL 451 2 internship)** Total Credits 17 Total