Drosophila Mode of Metamerization in the Embryogenesis of the Lepidopteran Insect Manduca Sexta
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Segmentation in Vertebrates: Clock and Gradient Finally Joined
Downloaded from genesdev.cshlp.org on September 24, 2021 - Published by Cold Spring Harbor Laboratory Press REVIEW Segmentation in vertebrates: clock and gradient finally joined Alexander Aulehla1 and Bernhard G. Herrmann2 Max-Planck-Institute for Molecular Genetics, Department of Developmental Genetics, 14195 Berlin, Germany The vertebral column is derived from somites formed by terior (A–P) axis. Somite formation takes place periodi- segmentation of presomitic mesoderm, a fundamental cally in a fixed anterior-to-posterior sequence. process of vertebrate embryogenesis. Models on the In the chick embryo, a new somite is formed approxi- mechanism controlling this process date back some mately every 90 min, whereas in the mouse embryo, the three to four decades. Access to understanding the mo- periodicity varies, dependent on the axial position (Tam lecular control of somitogenesis has been gained only 1981). Classical embryology experiments revealed that recently by the discovery of molecular oscillators (seg- periodicity and directionality of somite formation are mentation clock) and gradients of signaling molecules, controlled by an intrinsic program set off in the cells as as predicted by early models. The Notch signaling path- they are recruited into the psm. For instance, when the way is linked to the oscillator and plays a decisive role in psm is inverted rostro–caudally, somite formation in the inter- and intrasomitic boundary formation. An Fgf8 sig- inverted region proceeds from caudal to rostral, main- naling gradient is involved in somite size control. And taining the original direction (Christ et al. 1974). More- the (canonical) Wnt signaling pathway, driven by Wnt3a, over, neither the transversal bisection nor the isolation appears to integrate clock and gradient in a global of the psm from all surrounding tissues stops the seg- mechanism controlling the segmentation process. -

Perspectives
Copyright 0 1994 by the Genetics Society of America Perspectives Anecdotal, Historical and Critical Commentaries on Genetics Edited by James F. Crow and William F. Dove A Century of Homeosis, A Decade of Homeoboxes William McGinnis Department of Molecular Biophysics and Biochemistry, Yale University, New Haven, Connecticut 06520-8114 NE hundred years ago, while the science of genet- ing mammals, and were proposed to encode DNA- 0 ics still existed only in the yellowing reprints of a binding homeodomainsbecause of a faint resemblance recently deceased Moravian abbot, WILLIAMBATESON to mating-type transcriptional regulatory proteins of (1894) coined the term homeosis to define a class of budding yeast and an even fainter resemblance to bac- biological variations in whichone elementof a segmen- terial helix-turn-helix transcriptional regulators. tally repeated array of organismal structures is trans- The initial stream of papers was a prelude to a flood formed toward the identity of another. After the redis- concerning homeobox genes and homeodomain pro- coveryof MENDEL’Sgenetic principles, BATESONand teins, a flood that has channeled into a steady river of others (reviewed in BATESON1909) realized that some homeo-publications, fed by many tributaries. A major examples of homeosis in floral organs and animal skel- reason for the continuing flow of studies is that many etons could be attributed to variation in genes. Soon groups, working on disparate lines of research, have thereafter, as the discipline of Drosophila genetics was found themselves swept up in the currents when they born and was evolving into a formidable intellectual found that their favorite protein contained one of the force enriching many biologicalsubjects, it gradually be- many subtypes of homeodomain. -

PAPC Couples the Segmentation Clock to Somite Morphogenesis by Regulating N-Cadherin-Dependent Adhesion
© 2017. Published by The Company of Biologists Ltd | Development (2017) 144, 664-676 doi:10.1242/dev.143974 RESEARCH ARTICLE PAPC couples the segmentation clock to somite morphogenesis by regulating N-cadherin-dependent adhesion Jérome Chal1,2,3,4,5,*, Charlenè Guillot3,4,* and Olivier Pourquié1,2,3,4,5,6,7,‡ ABSTRACT specific level of the PSM called the determination front. The Vertebrate segmentation is characterized by the periodic formation of determination front is defined as a signaling threshold epithelial somites from the mesenchymal presomitic mesoderm implemented by posterior gradients of Wnt and FGF (Aulehla (PSM). How the rhythmic signaling pulse delivered by the et al., 2003; Diez del Corral and Storey, 2004; Dubrulle et al., segmentation clock is translated into the periodic morphogenesis of 2001; Hubaud and Pourquie, 2014; Sawada et al., 2001). Cells of somites remains poorly understood. Here, we focused on the role of the posterior PSM exhibit mesenchymal characteristics and paraxial protocadherin (PAPC/Pcdh8) in this process. We showed express Snail-related transcription factors (Dale et al., 2006; that in chicken and mouse embryos, PAPC expression is tightly Nieto, 2002). In the anterior PSM, cells downregulate snail/slug regulated by the clock and wavefront system in the posterior PSM. We expression and upregulate epithelialization-promoting factors such observed that PAPC exhibits a striking complementary pattern to N- as paraxis (Barnes et al., 1997; Sosic et al., 1997). This molecular cadherin (CDH2), marking the interface of the future somite boundary transition correlates with the anterior PSM cells progressively in the anterior PSM. Gain and loss of function of PAPC in chicken acquiring epithelial characteristics (Duband et al., 1987; Martins embryos disrupted somite segmentation by altering the CDH2- et al., 2009). -

133 Solanum Viarum Dunal (Solanaceae), Primer Reporte Para
Solanum viarum Dunal (Solanaceae), Primer Reporte para Honduras Rodrigo Díaz, Ana C. Samayoa y William A. Overholt1 Resumen. La maleza invasora Solanum viarum Dunal (Solanaceae) es reportada por primera vez para Honduras. La planta, nativa de Sudamérica, fue localizada en un estacionamiento de la Escuela Agrícola Panamericana, El Zamorano, Honduras, el 26 de noviembre de 2007. Esta maleza es altamente invasora en pasturas debido a que el ganado puede transportar semillas en su tracto digestivo. Palabras clave: Control biológico, maleza invasora, pasturas. Abstract. The invasive weed Solanum viarum Dunal (Solanaceae) is reported for first time in Honduras. This species, native to South America, was located in a parking lot at the Escuela Agrícola Panamericana, El Zamorano, Honduras, on November 26th, 2007. This weed is highly invasive in pastures due to cattle transporting seed in their digestive tract. Key words: Biological control, invasive weed, rangelands. Introducción es considerada una de las malezas más destructivas en zonas ganaderas. La planta se ha esparcido Descripción de la planta. Las plantas adultas de rápidamente debido en gran parte al transporte de Solanum viarum son arbustos de 1 – 2 m de alto con ganado desde Florida hacia los estados adyacentes espinas de hasta 3 cm de longitud en las hojas, (Ferrel y Mullahey 2006). Otras formas de transporte pecíolos y tallos. Las hojas y tallos son pubescentes y incluyen grama, heno y estiércol contaminados con de una contextura pegajosa; las flores son blancas con semillas. Si esta maleza no es controlada la cantidad de cinco pétalos recurvados y con estambres color cabezas de ganado por área es reducida, lo que sube amarillo (Ferrel y Mullahey 2006). -

Transformations of Lamarckism Vienna Series in Theoretical Biology Gerd B
Transformations of Lamarckism Vienna Series in Theoretical Biology Gerd B. M ü ller, G ü nter P. Wagner, and Werner Callebaut, editors The Evolution of Cognition , edited by Cecilia Heyes and Ludwig Huber, 2000 Origination of Organismal Form: Beyond the Gene in Development and Evolutionary Biology , edited by Gerd B. M ü ller and Stuart A. Newman, 2003 Environment, Development, and Evolution: Toward a Synthesis , edited by Brian K. Hall, Roy D. Pearson, and Gerd B. M ü ller, 2004 Evolution of Communication Systems: A Comparative Approach , edited by D. Kimbrough Oller and Ulrike Griebel, 2004 Modularity: Understanding the Development and Evolution of Natural Complex Systems , edited by Werner Callebaut and Diego Rasskin-Gutman, 2005 Compositional Evolution: The Impact of Sex, Symbiosis, and Modularity on the Gradualist Framework of Evolution , by Richard A. Watson, 2006 Biological Emergences: Evolution by Natural Experiment , by Robert G. B. Reid, 2007 Modeling Biology: Structure, Behaviors, Evolution , edited by Manfred D. Laubichler and Gerd B. M ü ller, 2007 Evolution of Communicative Flexibility: Complexity, Creativity, and Adaptability in Human and Animal Communication , edited by Kimbrough D. Oller and Ulrike Griebel, 2008 Functions in Biological and Artifi cial Worlds: Comparative Philosophical Perspectives , edited by Ulrich Krohs and Peter Kroes, 2009 Cognitive Biology: Evolutionary and Developmental Perspectives on Mind, Brain, and Behavior , edited by Luca Tommasi, Mary A. Peterson, and Lynn Nadel, 2009 Innovation in Cultural Systems: Contributions from Evolutionary Anthropology , edited by Michael J. O ’ Brien and Stephen J. Shennan, 2010 The Major Transitions in Evolution Revisited , edited by Brett Calcott and Kim Sterelny, 2011 Transformations of Lamarckism: From Subtle Fluids to Molecular Biology , edited by Snait B. -

Rapid Changes in Morphogen Concentration Control Self-Organized
RESEARCH COMMUNICATION Rapid changes in morphogen concentration control self-organized patterning in human embryonic stem cells Idse Heemskerk1†, Kari Burt1, Matthew Miller1, Sapna Chhabra2, M Cecilia Guerra1, Lizhong Liu1, Aryeh Warmflash1,3* 1Department of Biosciences, Rice University, Houston, United States; 2Systems, Synthetic and Physical Biology Program, Rice University, Houston, United States; 3Department of Bioengineering, Rice University, Houston, United States Abstract During embryonic development, diffusible signaling molecules called morphogens are thought to determine cell fates in a concentration-dependent way. Yet, in mammalian embryos, concentrations change rapidly compared to the time for making cell fate decisions. Here, we use human embryonic stem cells (hESCs) to address how changing morphogen levels influence differentiation, focusing on how BMP4 and Nodal signaling govern the cell-fate decisions associated with gastrulation. We show that BMP4 response is concentration dependent, but that expression of many Nodal targets depends on rate of concentration change. Moreover, in a self- organized stem cell model for human gastrulation, expression of these genes follows rapid changes in endogenous Nodal signaling. Our study shows a striking contrast between the specific ways ligand dynamics are interpreted by two closely related signaling pathways, highlighting both the *For correspondence: subtlety and importance of morphogen dynamics for understanding mammalian embryogenesis [email protected] and designing optimized protocols for directed stem cell differentiation. Editorial note: This article has been through an editorial process in which the authors decide how † Present address: Department to respond to the issues raised during peer review. The Reviewing Editor’s assessment is that all of Cell and Developmental the issues have been addressed (see decision letter). -

Defensive Sound Production in the Tobacco Hornworm, Manduca Sexta (Bombycoidea: Sphingidae)
J Insect Behav (2012) 25:114–126 DOI 10.1007/s10905-011-9282-8 Defensive Sound Production in the Tobacco Hornworm, Manduca sexta (Bombycoidea: Sphingidae) Veronica L. Bura & Antoine K. Hnain & Justin N. Hick & Jayne E. Yack Revised: 1 July 2011 /Accepted: 7 July 2011 / Published online: 21 July 2011 # Springer Science+Business Media, LLC 2011 Abstract The tobacco hornworm (Manduca sexta) is a model organism extensively studied for many aspects of its biology, including its anti-predator strategies. We report on a novel component of this caterpillar’sdefencerepertoire:sound production. Late instar caterpillars produce discrete clicking sounds in response to disturbance. Click trains range in duration from 0.3–20.0 s (mean 3.3±4.8 s) and contain 2–41 clicks (mean 7.1±9.5). Sounds are broadband with a dominant frequency of 29.8±4.9 kHz. We investigated the mechanism of sound production by selectively ablating three identified sets of ridges on the mandibles, and determined that ridges on the inner face strike the outer and incisor ridges on the opposing mandible to produce multi-component clicks. We tested the hypothesis that clicks function in defence using simulated attacks with blunt forceps. In single attack trials 77% of larvae produced sound and this increased to 100% in sequential attacks. Clicks preceded or accompanied regurgitation in 93% of multiple attack trials, indicating that sound production may function in acoustic aposematism. Sound production is also accompanied by other behaviours including directed thrashing, head curling, and biting, suggesting that sounds may also function as a general warning of unprofitability. -

Evaluating the Core Microbiome of Manduca Sexta Authors: Macy Johnson, Dr
Evaluating the Core Microbiome of Manduca sexta Authors: Macy Johnson, Dr. Jerreme Jackson*, and Dr. Tyrrell Conway† Abstract: Microbiomes are complex communities of microorganisms that colonize many surfaces of an animal’s body, especially those niches lined with carbohydrate-rich mucosal layers such as the eyes, male and female reproductive tracts, and the gastrointestinal tract. While a vast majority of data from microbiome studies has relied almost extensively on metagenomics-based approaches to identify individual species within these small complex communities, the contributions of these communities to host physiology remain poorly understood. We used a combination of culture- and non culture-based approaches to identify and begin functionally characterizing microbial inhabitants stably colonized in the midgut epithelium of the invertebrate model Manduca sexta (tobacco hornworm), an agriculture pest of Nicotiana attenuata (wild-tobacco) and many additional solanaceous plants. Keywords: Microbiome, Manduca sexta, Intestine, Nicotiana attenuata, Metagenomics Introduction supports the hypothesis that a core microbiome The animal intestinal microbiome comprises a persists in the intestinal tract of some Lepidopteran diverse community of microorganisms, which species. When the bacteria are transferred vertically, influence host development, physiology, and response they are passed on generationally. When the bacteria to pathogens. However, the mechanism underlying are transferred horizontally, they are passed directly these complex interactions -
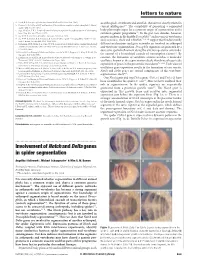
Involvement Ofnotchanddeltagenes in Spider Segmentation
letters to nature 4. Urick, R. J. Principles of Underwater Sound (McGraw Hill, New York, 1983). as arthropods, vertebrates and annelids, that are not closely related in 5. Henson, O. W. Jr The activity and function of the middle ear muscles in echolocating bats. J. Physiol. current phylogenies10. The complexity of generating a segmented (Lond.) 180, 871–887 (1965). 6. Suga, N. & Jen, P. H. S. Peripheral control of acoustic signals in the auditory system of echolocating body plan might argue for a common origin of segmentation and a bats. J. Exp. Biol. 62, 277–331 (1975). common genetic programme1,2. In the past two decades, however, 7. Au, W. W. L. The Sonar of Dolphins (Springer, New York, 1993). genetic analyses in the fruitfly Drosophila3,4 and in various vertebrates 8. Au, W. W. L., Ford, J. K. B. & Allman, K. A. Echolocation signals of foraging killer whales (Orcinus 7–9,11–14 orca). J. Acoust. Soc. Am. 111, 2343–2344 (2002). such as mouse, chick and zebrafish suggest that fundamentally 9. Rasmussen, M. H., Miller, L. A. & Au, W. W. L. Source levels of clicks from free-ranging white beaked different mechanisms and gene networks are involved in arthropod dolphins (Lagenorhynchus albirostris Gray 1846) recorded in Icelandic waters. J. Acoust. Soc. Am. 111, and vertebrate segmentation. Drosophila segments are generated by a 1122–1125 (2002). successive spatial refinement along the anterior–posterior axis under 10. Ketten, D. R. in Hearing by Whales and Dolphins (eds Au, W. W. L., Popper, A. N. & Fay, R. R.) 43–108 3,4 (Springer, New York, 2000). -

Tomato Hornworm Manduca Quinquemaculata (Haworth) (Insecta: Lepidoptera: Sphingidae) 1 Morgan A
EENY700 Tomato Hornworm Manduca quinquemaculata (Haworth) (Insecta: Lepidoptera: Sphingidae) 1 Morgan A. Byron and Jennifer L. Gillett-Kaufman2 Introduction uncommon in the Southeast and is replaced by the tobacco hornworm in this region. In Florida, hornworm damage on The tomato hornworm, Manduca quinquemacu- tomato is typically caused by the tobacco hornworm, rather lata (Haworth), is a common garden pest that feeds on than the tomato hornworm, despite its common name. plants in the Solanaceae (nightshade) family including tomato, peppers, eggplant, and potato. The adult form of the tomato hornworm is a relatively large, robust-bodied moth, commonly known as a hawk moth or sphinx moth. The adult moth feeds on the nectar of various flowers and, like the larval form, is most active from dusk until dawn (Lotts and Naberhaus 2017). The tomato hornworm (Figure 1) may be confused with the tobacco hornworm, Manduca sexta (L.) (Figure 2), a closely related species that also specializes on solanaceous plant species and is similar in Figure 1. Late instar larva of the tomato hornworm, Manduca appearance. Various morphological features can be used quinquemaculata (Haworth). to differentiate these hornworms, namely that tomato Credits: Paul Choate, UF/IFAS hornworm has V-shaped yellow-white markings on the body and the tobacco hornworm has white diagonal lines. Additionally, the horn, a small protrusion on the final abdominal segment of the caterpillar that gives the horn- worm its name, of the tomato hornworm is black, whereas the horn of the tobacco hornworm is reddish in color. Distribution The tomato hornworm has a wide distribution in North Figure 2. -

A Potential Biocontrol Agent of Tropical Soda Apple, Solanum Viarum (Solanaceae) in the USA
Risk assessment of Gratiana boliviana (Chrysomelidae), a potential biocontrol agent of tropical soda apple, Solanum viarum (Solanaceae) in the USA J. Medal,1,2 D. Gandolfo,3 F. McKay3 and J. Cuda1 Summary Solanum viarum (Solanaceae), known by the common name tropical soda apple, is a perennial prickly weed native to north-eastern Argentina, south-eastern Brazil, Paraguay, and Uruguay, that has been spreading at an alarming rate in the USA during the 1990s. First detected in the USA in 1988, it has already invaded more than 1 million acres (ca. 400,000 ha) of improved pastures and woody areas in nine states. Initial field explorations in South America for potential biocontrol agents were initiated in June 1994 by University of Florida researchers in collaboration with Brazilian and Argentinean scientists. The leaf beetle Gratiana boliviana (Chrysomelidae) was evaluated as a potential biocontrol agent of tropical soda apple. The only known hosts of this insect are S. viarum and Solanum palinacanthum. Open field experiments and field surveys were conducted to assess the risk of G. boliviana using Solanum melongena (eggplant) as an alternative host. In an open field (choice-test) planted with tropical soda apple and eggplant there was no feeding or oviposition by G. boliviana adults on eggplant. Surveys conducted (1997–2002) of 34 unsprayed fields of eggplant confirmed that this crop is not a host of G. boliviana. Based on these results, the Florida quarantine host-specificity tests, the open field tests in Argentina, and the lack of unfavourable host records in the scientific literature, we concluded that G. -

Stages of Embryonic Development of the Zebrafish
DEVELOPMENTAL DYNAMICS 2032553’10 (1995) Stages of Embryonic Development of the Zebrafish CHARLES B. KIMMEL, WILLIAM W. BALLARD, SETH R. KIMMEL, BONNIE ULLMANN, AND THOMAS F. SCHILLING Institute of Neuroscience, University of Oregon, Eugene, Oregon 97403-1254 (C.B.K., S.R.K., B.U., T.F.S.); Department of Biology, Dartmouth College, Hanover, NH 03755 (W.W.B.) ABSTRACT We describe a series of stages for Segmentation Period (10-24 h) 274 development of the embryo of the zebrafish, Danio (Brachydanio) rerio. We define seven broad peri- Pharyngula Period (24-48 h) 285 ods of embryogenesis-the zygote, cleavage, blas- Hatching Period (48-72 h) 298 tula, gastrula, segmentation, pharyngula, and hatching periods. These divisions highlight the Early Larval Period 303 changing spectrum of major developmental pro- Acknowledgments 303 cesses that occur during the first 3 days after fer- tilization, and we review some of what is known Glossary 303 about morphogenesis and other significant events that occur during each of the periods. Stages sub- References 309 divide the periods. Stages are named, not num- INTRODUCTION bered as in most other series, providing for flexi- A staging series is a tool that provides accuracy in bility and continued evolution of the staging series developmental studies. This is because different em- as we learn more about development in this spe- bryos, even together within a single clutch, develop at cies. The stages, and their names, are based on slightly different rates. We have seen asynchrony ap- morphological features, generally readily identi- pearing in the development of zebrafish, Danio fied by examination of the live embryo with the (Brachydanio) rerio, embryos fertilized simultaneously dissecting stereomicroscope.