1 Institution: Aarhus University, Department of Animal Science 1 2 Title: Quantitative Macroscopic Anatomy of the Giraffe (Giraf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Arctic Expedition12° 16° 20° 24° 28° 32° Spitsbergen U Svalbard Archipelago 80° 80°
distinguished travel for more than 35 years Voyage UNDER THE Midnight Sun Arctic Expedition12° 16° 20° 24° 28° 32° Spitsbergen u Svalbard Archipelago 80° 80° 80° Raudfjorden Nordaustlandet Woodfjorden Smeerenburg Monaco Glacier The Arctic’s 79° 79° 79° Kongsfjorden Svalbard King’s Glacier Archipelago Ny-Ålesund Spitsbergen Longyearbyen Canada 78° 78° 78° i Greenland tic C rcle rc Sea Camp Millar A U.S. North Pole Russia Bellsund Calypsobyen Svalbard Archipelago Norway Copenhagen Burgerbukta 77° 77° 77° Cruise Itinerary Denmark Air Routing Samarin Glacier Hornsund Barents Sea June 20 to 30, 2022 4° 8° Spitsbergen12° u Samarin16° Glacier20° u Calypsobyen24° 76° 28° 32° 36° 76° Voyage across the Arctic Circle on this unique 11-day Monaco Glacier u Smeerenburg u Ny-Ålesund itinerary featuring a seven-night cruise round trip Copenhagen 1 Depart the U.S. or Canada aboard the Five-Star Le Boréal. Visit during the most 2 Arrive in Copenhagen, Denmark enchanting season, when the region is bathed in the magical 3 Copenhagen/Fly to Longyearbyen, Spitsbergen, light of the Midnight Sun. Cruise the shores of secluded Norway’s Svalbard Archipelago/Embark Le Boréal 4 Hornsund for Burgerbukta/Samarin Glacier Spitsbergen—the jewel of Norway’s rarely visited Svalbard 5 Bellsund for Calypsobyen/Camp Millar archipelago enjoy expert-led Zodiac excursions through 6 Cruising the Arctic Ice Pack sandstone mountain ranges, verdant tundra and awe-inspiring 7 MåkeØyane/Woodfjorden/Monaco Glacier ice formations. See glaciers calve in luminous blues and search 8 Raudfjorden for Smeerenburg for Arctic wildlife, including the “King of the Arctic,” the 9 Ny-Ålesund/Kongsfjorden for King’s Glacier polar bear, whales, walruses and Svalbard reindeer. -

Browsing and Non-Browsing Extant and Extinct Giraffids Evidence From
Browsing and non-browsing extant and extinct giraffids Evidence from dental microwear textural analysis Gildas Merceron, Marc Colyn, Denis Geraads To cite this version: Gildas Merceron, Marc Colyn, Denis Geraads. Browsing and non-browsing extant and extinct giraffids Evidence from dental microwear textural analysis. Palaeogeography, Palaeoclimatology, Palaeoecol- ogy, Elsevier, 2018, 505, pp.128-139. 10.1016/j.palaeo.2018.05.036. hal-01834854v2 HAL Id: hal-01834854 https://hal-univ-rennes1.archives-ouvertes.fr/hal-01834854v2 Submitted on 6 Sep 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 Browsing and non-browsing extant and extinct giraffids: evidence from dental microwear 2 textural analysis. 3 4 Gildas MERCERON1, Marc COLYN2, Denis GERAADS3 5 6 1 Palevoprim (UMR 7262, CNRS & Université de Poitiers, France) 7 2 ECOBIO (UMR 6553, CNRS & Université de Rennes 1, Station Biologique de Paimpont, 8 France) 9 3 CR2P (UMR 7207, Sorbonne Universités, MNHN, CNRS, UPMC, France) 10 11 1Corresponding author: [email protected] 12 13 Abstract: 14 15 Today, the family Giraffidae is restricted to two genera endemic to the African 16 continent, Okapia and Giraffa, but, with over ten genera and dozens of species, it was far 17 more diverse in the Old World during the late Miocene. -

Climate in Svalbard 2100
M-1242 | 2018 Climate in Svalbard 2100 – a knowledge base for climate adaptation NCCS report no. 1/2019 Photo: Ketil Isaksen, MET Norway Editors I.Hanssen-Bauer, E.J.Førland, H.Hisdal, S.Mayer, A.B.Sandø, A.Sorteberg CLIMATE IN SVALBARD 2100 CLIMATE IN SVALBARD 2100 Commissioned by Title: Date Climate in Svalbard 2100 January 2019 – a knowledge base for climate adaptation ISSN nr. Rapport nr. 2387-3027 1/2019 Authors Classification Editors: I.Hanssen-Bauer1,12, E.J.Førland1,12, H.Hisdal2,12, Free S.Mayer3,12,13, A.B.Sandø5,13, A.Sorteberg4,13 Clients Authors: M.Adakudlu3,13, J.Andresen2, J.Bakke4,13, S.Beldring2,12, R.Benestad1, W. Bilt4,13, J.Bogen2, C.Borstad6, Norwegian Environment Agency (Miljødirektoratet) K.Breili9, Ø.Breivik1,4, K.Y.Børsheim5,13, H.H.Christiansen6, A.Dobler1, R.Engeset2, R.Frauenfelder7, S.Gerland10, H.M.Gjelten1, J.Gundersen2, K.Isaksen1,12, C.Jaedicke7, H.Kierulf9, J.Kohler10, H.Li2,12, J.Lutz1,12, K.Melvold2,12, Client’s reference 1,12 4,6 2,12 5,8,13 A.Mezghani , F.Nilsen , I.B.Nilsen , J.E.Ø.Nilsen , http://www.miljodirektoratet.no/M1242 O. Pavlova10, O.Ravndal9, B.Risebrobakken3,13, T.Saloranta2, S.Sandven6,8,13, T.V.Schuler6,11, M.J.R.Simpson9, M.Skogen5,13, L.H.Smedsrud4,6,13, M.Sund2, D. Vikhamar-Schuler1,2,12, S.Westermann11, W.K.Wong2,12 Affiliations: See Acknowledgements! Abstract The Norwegian Centre for Climate Services (NCCS) is collaboration between the Norwegian Meteorological In- This report was commissioned by the Norwegian Environment Agency in order to provide basic information for use stitute, the Norwegian Water Resources and Energy Directorate, Norwegian Research Centre and the Bjerknes in climate change adaptation in Svalbard. -

Arctic Environments
Characteristics of an arctic environment and the physical geography of Svalbard - ‘geography explained’ fact sheet The Arctic environment is little studied at Key Stage Three yet it is an excellent basis for an all-encompassing study of place or as a case study to illustrate key concepts within a specific theme. Svalbard, an archipelago lying in the Arctic Ocean north of mainland Europe, about midway between Norway and the North Pole, is a place with an awesome landscape and unique geography that includes issues and themes of global, regional and local importance. A study of Svalbard could allow pupils to broaden and deepen their knowledge and understanding of different aspects of the seven geographical concepts that underpin the revised Geography Key Stage Three Programme of Study. Many pupils will have a mental image of an Arctic landscape, some may have heard of Svalbard. A useful starting point for study is to explore these perceptions using visual prompts and big questions – where is the Arctic/Svalbard? What is it like? What is happening there? Why is it like this? How will it change? Svalbard exemplifies the distinctive physical and human characteristics of the Arctic and yet is also unique amongst Arctic environments. Perceptions and characteristics of the Arctic may be represented in many ways, including art and literature and the pupil’s own geographical imagination of the place. Maps and photographs are vital in helping pupils develop spatial understanding of locations, places and processes and the scale at which they occur. Source: commons.wikimedia.org/wiki/Image:W_W_Svalbard... 1 Longyearbyen, Svalbard’s capital Source:http://www.photos- The landscape of Western Svalbard voyages.com/spitzberg/images/spitzberg06_large.jpg Source: www.hi.is/~oi/svalbard_photos.htm Where is Svalbard? Orthographic map projection centred on Svalbard and showing location relative to UK and EuropeSource: www.answers.com/topic/orthographic- projection.. -

Youth Guide to Biodiversity
SPECIES ECOSYSTEMS HABITATS SOILS CLOUDS LAKES RIVERS MOUNTAINS HUMANS INSECTS MAMMALS OCEANS BIRDS FISHES WORMS PLANTS FLOWERS BEES ACTIONS HABITS ORGANIZATIONS SPECIES ECOSYSTEMS HABITATS SOILS CLOUDS LAKES RIVERS MOUNTAINS HUMANS INSECTS MAMMALS OCEANS BIRDS FISHES WORMS PLANTS FLOWERS BEES ACTIONS HABITS ORGANIZATIONS SPECIES ECOSYSTEMS HABITATS SOILS CLOUDS LAKES RIVERS MOUNTAINS HUMANS INSECTS YOUTH AND UNITED NATIONS GLOBAL ALLIANCE LEARNING AND ACTION SERIES MAMMALS OCEANS BIRDS FISHES WORMS PLANTS FLOWERS BEES ACTIONS HABITS ORGANIZATIONS SPECIES ECOSYSTEMS HABITATS SOILS CLOUDS LAKES RIVERS YOUTH AND UNITED NATIONS GLOBAL ALLIANCE LEARNING AND ACTION SERIES SUPPORTED BY MOUNTAINS HUMANS INSECTS MAMMALS OCEANS BIRDS FISHES WORMS PLANTS FLOWERS BEES ACTIONS HABITS ORGANIZATIONS SPECIES ECOSYSTEMS HABITATS TAKE ACTION FOR BIODIVERSITY! THE TO SOILS CLOUDS LAKES RIVERS MOUNTAINS HUMANS INSECTS MAMMALS OCEANS BIRDS FISHES WORMS PLANTS FLOWERS BEES ACTIONS HABITS ORGANIZATIONS SPECIES ECOSYSTEMS HABITATS SOILS CLOUDS LAKES RIVERS MOUNTAINS HUMANS INSECTS MAMMALS OCEANS BIRDS FISHES WORMS PLANTS FLOWERS BEES YOUTHGUIDE BIODIVERSITY ACTIONS HABITSWE ORGANIZATIONS ARE SPECIES ECOSYSTEMS MANY HABITATS SOILS CLOUDS LAKES RIVERS MOUNTAINS HUMANS INSECTS MAMMALS OCEANS BIRDS FISHES WORMS PLANTS FLOWERS BEES ACTIONS HABITS ORGANIZATIONS SPECIES ECOSYSTEMS HABITATS SOILS CLOUDS LAKES RIVERS MOUNTAINS HUMANS INSECTS MAMMALS OCEANS BIRDS FISHES WORMS PLANTS FLOWERS BEES ACTIONS HABITS ORGANIZATIONSwww. SPECIES ECOSYSTEMS HABITATS SOILS CLOUDS -

Wildlife and Wild Places in Mozambique K
Wildlife and Wild Places in Mozambique K. L Tinley, A. J. Rosinha, Jose L. P. Lobao Tello and T. P. Dutton This account of the national parks, reserves and other places worthy of pro- tection in Mozambique gives some idea of the wealth of wildlife in this newly independent country. One special reserve has 25,000 buffaloes—the largest concentration in the world. Protected conservation areas in Mozambique fall into six categories: 1. Parques nacionais - national parks; 2. Reservas especiais - special game reserves; 3. Reservas parciais - partial reserves; 4. Regimen de vigilancia - fauna protection zones; 5. Coutadas - hunting and photographic safari areas, normally run on a private concession basis; 6. Reservas florestais - forest reserves. Some unique areas are still outside this system but have been recommended for inclusion, together with other ecosystems worthy of inclusion in the future. Game farming or ranching is attracting considerable interest; one private and one government scheme have been proposed. NATIONAL PARKS 1.* Parque Nacional da Gorongosa (c. 3770 sq km). Situated at the southern limit of the great rift valley with an extensive flood plain and associated lakes, this park includes Brachystegia woodland Acacia and Combretum savanna. Sharply rising inselbergs (volcanic protrusions) are also a feature. The ungulates are typical floodplain species, including elephant (abundant), buffalo, wildebeest, waterbuck, zebra, reedbuck, impala and oribi; on the elevated woodland and savanna habitat there are black rhino, eland, Lichten- stein's hartebeest, sable, kudu, nyala, Sharpe's grysbok, suni, blue and grey duiker, and klipspringer are common on rock outcrops; lion, leopard and hippopotamus are abundant. Both land and water birds are prolific and diverse, and crocodiles are very common. -
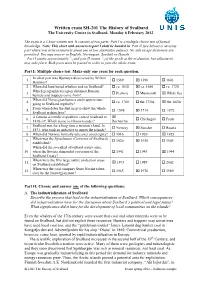
Written Exam SH-201 the History of Svalbard the University Centre in Svalbard, Monday 6 February 2012
Written exam SH-201 The History of Svalbard The University Centre in Svalbard, Monday 6 February 2012 The exam is a 3 hour written test. It consists of two parts: Part I is a multiple choice test of factual knowledge. Note: This sheet with answers to part I shall be handed in. Part II (see below) is an essay part where you write extensively about one of two alternative subjects. No aids except dictionary are permitted. You may answer in English, Norwegian, Swedish or Danish. 1 2 Part I counts approximately /3 and part II counts /3 of the grade at the evaluation, but adjustment may take place. Both parts must be passed in order to pass the whole exam. Part I: Multiple choice test. Make only one cross for each question. In what year was Bjørnøya discovered by Willem 1. 1569 1596 1603 Barentsz? 2. When did land-based whaling end on Svalbard? ca. 1630 ca. 1680 ca. 1720 Which geographical region did most Russian 3. Pechora Murmansk White Sea hunters and trappers come from? When did Norwegian hunters and trappers start 4. ca. 1700 the 1750s the 1820s going to Svalbard regularly? From when dates the first map to show the whole 5. 1598 1714 1872 Svalbard archipelago? A famous scientific expedition visited Svalbard in 6. Chichagov Fram 1838–39. Which name is it known under? Recherche Svalbard was for a long time a no man’s land. In 7. Norway Sweden Russia 1871, who took an initiative to annex the islands? 8. When did Norway formally take over sovereignty? 1916 1920 1925 When was the Sysselmann (Governor of Svalbard) 9. -

South Africa and Namibia, 20 July - 12 August 2017
South Africa and Namibia, 20 July - 12 August 2017. VLADIMIR DINETS This trip was an introduction to Africa for my wife and daughter; the latter was two and a half years old at the time and enjoyed it a lot. We had three weeks to kill while a container with our stuff was traveling from New Jersey to our new home in Okinawa. We started from Cape Town, drove east to Hermanus and then north to Oranjemund, flew to Windhoek, briefly explored a few locations up to Etosha, returned to Windhoek and spent one day and two nights in Johannesburg. In 2017, Cape Town area, Namaqualand and Namibia were experiencing a major drought. Full moon was on August 7; that made finding small mammals difficult during the Namibian part of the trip. I had six Sherman traps and caught only twelve rodents of two species (two pygmy mice in the far south and Namaqua rock rats almost everywhere else). Bats seemed absent with few exceptions. I saw almost a hundred species, all but four of which I had seen before (see my 2008 trip report), and removed a few species from my “better view much desired” list. South Africa Simon’s Town is a convenient base for exploring the area; M4 south from it is a good place to look for chacma baboons, while humpback whales are often feeding offshore (we saw one, and heard a few while scuba diving). Watch for South African fur seals, too. Four-striped grass mice (locally called “field mice”) were abundant in wooded parts of penguin colonies. -

Australia's Biodiversity and Climate Change
Australia’s Biodiversity and Climate Change A strategic assessment of the vulnerability of Australia’s biodiversity to climate change A report to the Natural Resource Management Ministerial Council commissioned by the Australian Government. Prepared by the Biodiversity and Climate Change Expert Advisory Group: Will Steffen, Andrew A Burbidge, Lesley Hughes, Roger Kitching, David Lindenmayer, Warren Musgrave, Mark Stafford Smith and Patricia A Werner © Commonwealth of Australia 2009 ISBN 978-1-921298-67-7 Published in pre-publication form as a non-printable PDF at www.climatechange.gov.au by the Department of Climate Change. It will be published in hard copy by CSIRO publishing. For more information please email [email protected] This work is copyright. Apart from any use as permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission from the Commonwealth. Requests and inquiries concerning reproduction and rights should be addressed to the: Commonwealth Copyright Administration Attorney-General's Department 3-5 National Circuit BARTON ACT 2600 Email: [email protected] Or online at: http://www.ag.gov.au Disclaimer The views and opinions expressed in this publication are those of the authors and do not necessarily reflect those of the Australian Government or the Minister for Climate Change and Water and the Minister for the Environment, Heritage and the Arts. Citation The book should be cited as: Steffen W, Burbidge AA, Hughes L, Kitching R, Lindenmayer D, Musgrave W, Stafford Smith M and Werner PA (2009) Australia’s biodiversity and climate change: a strategic assessment of the vulnerability of Australia’s biodiversity to climate change. -

A Scoping Review of Viral Diseases in African Ungulates
veterinary sciences Review A Scoping Review of Viral Diseases in African Ungulates Hendrik Swanepoel 1,2, Jan Crafford 1 and Melvyn Quan 1,* 1 Vectors and Vector-Borne Diseases Research Programme, Department of Veterinary Tropical Disease, Faculty of Veterinary Science, University of Pretoria, Pretoria 0110, South Africa; [email protected] (H.S.); [email protected] (J.C.) 2 Department of Biomedical Sciences, Institute of Tropical Medicine, 2000 Antwerp, Belgium * Correspondence: [email protected]; Tel.: +27-12-529-8142 Abstract: (1) Background: Viral diseases are important as they can cause significant clinical disease in both wild and domestic animals, as well as in humans. They also make up a large proportion of emerging infectious diseases. (2) Methods: A scoping review of peer-reviewed publications was performed and based on the guidelines set out in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension for scoping reviews. (3) Results: The final set of publications consisted of 145 publications. Thirty-two viruses were identified in the publications and 50 African ungulates were reported/diagnosed with viral infections. Eighteen countries had viruses diagnosed in wild ungulates reported in the literature. (4) Conclusions: A comprehensive review identified several areas where little information was available and recommendations were made. It is recommended that governments and research institutions offer more funding to investigate and report viral diseases of greater clinical and zoonotic significance. A further recommendation is for appropriate One Health approaches to be adopted for investigating, controlling, managing and preventing diseases. Diseases which may threaten the conservation of certain wildlife species also require focused attention. -

Zimbabwe & Botswana TRIP REPORT Nov 2017 Summary
ZIMBABWE & BOTSWANA WILDLIFE SAFARI November 3-15, 2017 TRIP REPORT Summary by Adrian Binns Mana Pools, Zimbabwe Our 12-day African safari began on the eastern edge of Mana Pools National Park in northern Zimbabwe. From our bush camps at Ruckomechi and neighbouring Little Ruckomechi, we enjoyed exploring a range of habitats teeming with wonderful wildlife. Elephants were among the first to greet us, as they roamed freely near the tents, even venturing next to the swimming pool! We kept a watchful eye and careful distance, in awe of the giant pachyderms. What a welcome treat! Our camps overlooked the mighty Zambezi river towards Zambia’s Rift Valley escarpment, providing excellent opportunity for relaxing afternoon boat rides. We drifted slowly up to wallowing pods of hippos, basking crocodiles, and African Skimmers loafing on exposed narrow sand strips. In steeply-eroded river banks, White- fronted and Southern Carmine Bee-eaters excavated their tunnel nests. The location helps keep predators at bay, but not all of them! On two consecutive afternoons we watched a Nile Water Monitor dig into the tunnels with its long sharp claws, and come out with a youngster, much to the chagrin of the parents. We watched elephants trek through tall grasses of river islands, and cross channels to reach the opposite bank. We cheered when one struggling baby elephant finally made it across. One evening we were moored on a riverbank enjoying sundowners, when an inquisitive elephant approached to within feet of Jane who was seated at the front of the boat. It was a heart-stopping moment to be face-to-face with such a huge animal – so close we could count the eyelashes – but we had nowhere to go. -

Samburu National Reserve Lake Nakuru National Park
EAST AFRICAN BIRDING EAST AFRICAN BIRDING Herds of zebras and wildebeest grazing endless grasslands studded with flat- Samburu NR Shaba NR SOMALIA topped acacia trees, dramatic volcanic UGANDA Buffalo Springs NR calderas brimming with big game and Kakamega Forest predators, red-robed Maasai herding Mt Kenya NP eastern Mt Kenya skinny cattle – these are well-known images of quintessential Africa, and Lake Victoria Lake Nakuru NP they can all be discovered in Kenya and KENYA Tanzania. Less well known is that each Nairobi Masai Mara NR country hosts more than 1 000 bird species. This diversity, combined with a superb network of protected areas, ex- Serengeti NP PROMISE Amboseli NR cellent lodges and friendly people, prompts Adam Riley to recommend them as top birding destinations. Although there are many excellent locations within each nation – think Masai Mara, Amboseli and Kakamega in Kenya and Ngorongoro Mt Kilimanjaro Crater Selous, the Eastern Arc Mountains and Zanzibar in Tanzania – he describes here just six that shouldn’t be missed. • Arusha They can all be visited in one two-week trip, starting in Nairobi and ending in Arusha. Any time of year is good, even TANZANIA the April–May rainy season when the scenery is lush and there is less dust, and when there are fewer tourists and • Mombasa N Tarangire NP rates are lower. Expect to net about 450 bird and more than 50 mammal species on an adventure to these parks. U TEXT & PHOTOGRAPHS BY ADAM RILEY INDIAN 200 km OCEAN The arid, acacia-studded savanna of Samburu, Shaba and Buffalo Springs national reserves are SAMBURU NATIONAL interspersed with rugged hill ranges.