Crane Husbandry Manual 1999
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
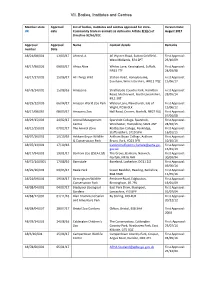
VII. Bodies, Institutes and Centres
VII. Bodies, Institutes and Centres Member state Approval List of bodies, institutes and centres approved for intra- Version Date: UK date Community trade in animals as defined in Article 2(1)(c) of August 2017 Directive 92/65/EEC Approval Approval Name Contact details Remarks number Date AB/21/08/001 13/03/17 Ahmed, A 46 Wyvern Road, Sutton Coldfield, First Approval: West Midlands, B74 2PT 23/10/09 AB/17/98/026 09/03/17 Africa Alive Whites Lane, Kessingland, Suffolk, First Approval: NR33 7TF 24/03/98 AB/17/17/005 15/06/17 All Things Wild Station Road, Honeybourne, First Approval: Evesham, Worcestershire, WR11 7QZ 15/06/17 AB/78/14/002 15/08/16 Amazonia Strathclyde Country Park, Hamilton First Approval: Road, Motherwell, North Lanarkshire, 28/05/14 ML1 3RT AB/29/12/003 06/04/17 Amazon World Zoo Park Watery Lane, Newchurch, Isle of First Approval: Wight, PO36 0LX 15/06/12 AB/17/08/065 08/03/17 Amazona Zoo Hall Road, Cromer, Norfolk, NR27 9JG First Approval: 07/04/08 AB/29/15/003 24/02/17 Animal Management Sparsholt College, Sparsholt, First Approval: Centre Winchester, Hampshire, SO21 2NF 24/02/15 AB/12/15/001 07/02/17 The Animal Zone Rodbaston College, Penkridge, First Approval: Staffordshire, ST19 5PH 16/01/15 AB/07/16/001 10/10/16 Askham Bryan Wildlife Askham Bryan College, Askham First Approval: & Conservation Park Bryan, York, YO23 3FR 10/10/16 AB/07/13/001 17/10/16 [email protected]. First Approval: gov.uk 15/01/13 AB/17/94/001 19/01/17 Banham Zoo (ZSEA Ltd) The Grove, Banham, Norwich, First Approval: Norfolk, NR16 -

Verzeichnis Der Europäischen Zoos Arten-, Natur- Und Tierschutzorganisationen
uantum Q Verzeichnis 2021 Verzeichnis der europäischen Zoos Arten-, Natur- und Tierschutzorganisationen Directory of European zoos and conservation orientated organisations ISBN: 978-3-86523-283-0 in Zusammenarbeit mit: Verband der Zoologischen Gärten e.V. Deutsche Tierpark-Gesellschaft e.V. Deutscher Wildgehege-Verband e.V. zooschweiz zoosuisse Schüling Verlag Falkenhorst 2 – 48155 Münster – Germany [email protected] www.tiergarten.com/quantum 1 DAN-INJECT Smith GmbH Special Vet. Instruments · Spezial Vet. Geräte Celler Str. 2 · 29664 Walsrode Telefon: 05161 4813192 Telefax: 05161 74574 E-Mail: [email protected] Website: www.daninject-smith.de Verkauf, Beratung und Service für Ferninjektionsgeräte und Zubehör & I N T E R Z O O Service + Logistik GmbH Tranquilizing Equipment Zootiertransporte (Straße, Luft und See), KistenbauBeratung, entsprechend Verkauf undden Service internationalen für Ferninjektionsgeräte und Zubehör Vorschriften, Unterstützung bei der Beschaffung der erforderlichenZootiertransporte Dokumente, (Straße, Vermittlung Luft und von See), Tieren Kistenbau entsprechend den internationalen Vorschriften, Unterstützung bei der Beschaffung der Celler Str.erforderlichen 2, 29664 Walsrode Dokumente, Vermittlung von Tieren Tel.: 05161 – 4813192 Fax: 05161 74574 E-Mail: [email protected] Str. 2, 29664 Walsrode www.interzoo.deTel.: 05161 – 4813192 Fax: 05161 – 74574 2 e-mail: [email protected] & [email protected] http://www.interzoo.de http://www.daninject-smith.de Vorwort Früheren Auflagen des Quantum Verzeichnis lag eine CD-Rom mit der Druckdatei im PDF-Format bei, welche sich großer Beliebtheit erfreute. Nicht zuletzt aus ökologischen Gründen verzichten wir zukünftig auf eine CD-Rom. Stattdessen kann das Quantum Verzeichnis in digitaler Form über unseren Webshop (www.buchkurier.de) kostenlos heruntergeladen werden. Die Datei darf gerne kopiert und weitergegeben werden. -

Quantifying Crop Damage by Grey Crowned Crane Balearica
QUANTIFYING CROP DAMAGE BY GREY CROWNED CRANE BALEARICA REGULORUM REGULORUM AND EVALUATING CHANGES IN CRANE DISTRIBUTION IN THE NORTH EASTERN CAPE, SOUTH AFRICA. By MARK HARRY VAN NIEKERK Department of the Zoology and Entomology, Rhodes University Submitted in partial fulfilment of the requirements for the Degree of MASTER OF SCIENCE December 2010 Supervisor: Prof. Adrian Craig i TABLE OF CONTENTS List of tables…………………………………………………………………………iv List of figures ………………………………………………………………………...v Abstract………………………………………………………………………………vii I. INTRODUCTION .......................................................................................... 1 Species account......................................................................................... 3 Habits and diet ........................................................................................... 5 Use of agricultural lands by cranes ............................................................ 6 Crop damage by cranes ............................................................................. 7 Evaluating changes in distribution and abundance of Grey Crowned Crane………………………………………………………..9 Objectives of the study………………………………………………………...12 II. STUDY AREA…………………………………………………………………...13 Locality .................................................................................................... 13 Climate ..................................................................................................... 15 Geology and soils ................................................................................... -

Rigenerazione, Ricostruzione, Recupero, Riuso, Resilienza
Maurizio Carta, Andrea Arcidiacono, 03.MicheleTalia, Carlo Gasparrini, Stefano Stanghellini, Carolina Giaimo, Francesco Sbetti Rigenerazione, OLTRE L'EMERGENZA. Un nuovo approccio alla ricostruzione, pianificazione dei territori recupero, riuso, a rischio. Francesco Alberti, Roberto Fiaschi, resilienza Marco Natali e Francesca Tommasoni* Introduzione Nel 2015 il forum di Parigi sui Cambiamenti Il presente contributo illustra gli esiti princi- Climatici “COP 21” e la pubblicazione dell’A- pali di una ricerca nel campo del Pre- e genda ONU recante gli Obiettivi di sviluppo Post-Disaster Recovery Planning, svilup- sostenibile per il 2030 (Sustainable Deve- pata in relazione agli assi prioritari III e IV lopment Goals - SDGs) hanno posto l’accen- della Chart of Sendai Framwork all’interno to, tra le altre cose, sulla necessità di rendere del Dipartimento di Architettura di Firen- le città e i territori più resilienti ai grandi di- ze con la collaborazione del Dipartimento sastri naturali. Sul tema, nello stesso anno si di Protezione Civile della Regione Toscana è inoltre tenuta a Sendai (Giappone) la “3rd e dell’ufficio della Protezione Civile dell’U- UN World Conference on Disaster Risk Re- nione dei Comuni della Garfagnana (1). Più duction”, che ha portato alla sottoscrizione specificamente, la ricerca ha inteso perse- da parte dei paesi membri di una carta d’in- guire tre obiettivi connessi alle diverse fasi tenti - The Chart of Sendai Framework – nel- di pianificazione/progettazione delle aree di la quale sono stati fissati gli obiettivi globali emergenza che, ai sensi della legislazione vi- di riduzione dei rischi e dei danni connessi a gente in Italia, gli enti territoriali sono tenu- eventi calamitosi in termini di popolazione ti a reperire ai fini della gestione di possibili esposta, vittime, perdite economiche, etc., da calamità. -

Our Brochure
Fifty years ago our National Bird, the Blue Crane, was a common sight in South Africa's grasslands, today it is rare. The KZN Crane Foundation, was formed to understand why and find ways to reverse this trend. inside front cover The KZN Crane Foundation By the year 2000, the number of Blue Cranes in the grasslands of the Eastern Cape, KZN, the Free State, Gauteng, and Mpumalanga, had declined by 90%. Their breeding requirements are, however, flexible and they have been able to adapt to new habitats in the Karoo and the wheat-lands of the Southern Cape and the total national population appears, for now, although greatly reduced, to be stable. Wattled Cranes (our largest and most regal species), unlike Blue Cranes, are much more specific about their nesting requirements (needing wetlands) and have been unable to adapt to other habitats. Like the Blue Cranes their population plummeted during the Nineteen eighties and nineties and by the year 2000, only 80 breeding pairs remained, an almost unsustainable level. At the time, their extinction seemed inevitable. In light of this, in 1989 concerned conservationists, under the leadership of the then Natal Parks Board, formed the KZN Crane Foundation with the aim of understanding and reversing this decline. (Photo Thanks to Daniel Dolpire) Are Cranes Important? Cranes are ancient birds whose elegance and beauty have long captured man's imagination, with references to them in the bible and the mythologies of many cultures, as symbols of peace, fidelity, and longevity. South African legend has it that Shaka wore Blue Crane feathers in his head-dress and decreed that feathers of these magnificent birds may be worn only by kings. -

Characterization of Gizzards and Grits of Wild Cranes Found Dead at Izumi Plain in Japan
FULL PAPER Wildlife Science Characterization of gizzards and grits of wild cranes found dead at Izumi Plain in Japan Mima UEGOMORI1), Yuko HARAGUCHI2), Takeshi OBI3) and Kozo TAKASE3)* 1)Laboratory of Veterinary Microbiology, Department of Veterinary Medicine, Faculty of Agriculture, Kagoshima University, 1-21-24 Korimoto, Kagoshima 890-0065, Japan 2)Izumi City Crane Museum, Crane Park Izumi, Izumi, Kagoshima 899-0208, Japan 3)Department of Veterinary Medicine, Joint Faculty of Veterinary Medicine, Kagoshima University, 1-21-24 Korimoto, Kagoshima 890-0065, Japan ABSTRACT. We analyzed the gizzards, and grits retained in the gizzards of 41 cranes that migrated to the Izumi Plain during the winter of 2015/2016 and died there, either due to accident or disease. These included 31 Hooded Cranes (Grus monacha) and 10 White-naped Cranes (G. vipio). We determined body weight, gizzard weight, total grit weight and number per gizzard, and size, shape, and surface roundness of the grits. Average gizzard weights were 92.4 g for Hooded Cranes and 97.1 g for White-naped Cranes, and gizzard weight positively correlated with J. Vet. Med. Sci. body weight in both species. Average total grit weights per gizzard were 19.7 g in Hooded Cranes 80(4): 642–647, 2018 and 25.7 g in White-naped Cranes, and were significantly higher in the latter. Average percentages of body weight to grit weight were 0.8% in Hooded Cranes and 0.5% in White-naped Cranes. doi: 10.1292/jvms.17-0407 Average grit number per gizzard was 693.5 in Hooded Cranes and 924.2 in White-naped Cranes, and were significantly higher in the latter. -

Demoiselle Cranes (Anthropoides Virgo) — an Attempt at Survival
DEMOISELLE CRANES (ANTHROPOIDES VIRGO) — AN ATTEMPT AT SURVIVAL Drs. Joost A. van der Ven The Netherlands If you wish to become crane-minded, watch the Demoiselle crane, and you will be lost for ever. That is, if you can find the Demoiselle in its natural habitat: breeding in the dry of the steppe; resting by a dried lakeside during the night or wintering in the green fields of the tropical regions. The Demoiselle crane is not 'endangered'. Their numbers are not that low, but each crane species, and almost all bird species will be endangered if we continue to build, to farm, to electrify, to hunt and to drain as we have done in the last hundred years. The crane habitat is important for so many other birds that we should pay much more attention to these birds than to many others. The cranes are by no means the easiest birds to ensure protection for, but if we achieve success here, there will be important habitat provided for many other bird species and animals. The protection (or wise use) of their habitats means a continuous battle against all who want to use these areas for other purposes. We don't want to be losers again, as many areas have been in the past and the remaining areas are needed for breeding, wintering and step- ping stones between them. The photographs of Bengt Berg taken in the thirties along the river Nile show us flocks of Demoiselle cranes in an area where nowadays the cranes have gone. The flocks of wintering cranes in Gujarat (India) seem to be smaller than those of several years ago. -

Conservation Measures for the Siberian Crane
CMS Technical Series Publication No. 1 Conservation Measures for the Siberian Crane Convention on Migratory Species Published by: UNEP/CMS Secretariat, Bonn, Germany Recommended citation: UNEP/CMS. ed.(1999). Conservation Measures for the Siberian Crane. CMS Technical Series Publication No.1, UNEP/CMS Secretariat, Bonn, Germany. Cover photograph: Siberian Crane (Grus leucogeranus) in snow. © Sietre / BIOS, Paris © UNEP/CMS, 1999 (copyright of individual contributions remains with the authors). Reproduction of this publication, except the cover photograph, for educational and other non-commercial purposes is authorized without permission from the copyright holder, provided the source is cited and the copyright holder receives a copy of the reproduced material. Reproduction of the text for resale or other commercial purposes, or of the cover photograph, is prohibited without prior permission of the copyright holder. The views expressed in this publication are those of the authors and do not necessarily reflect the views or policies of UNEP/CMS, nor are they an official record. The designation of geographical entities in this publication, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of UNEP/CMS concerning the legal status of any country, territory or area, or of its authorities, nor concerning the delimitation of its frontiers and boundaries. Copies of this publication are available from the UNEP/CMS Secretariat, United Nations Premises in Bonn, Martin-Luther-King-Str. 8, D-53175 -

Modelling Risk of Blue Crane (Anthropoides Paradiseus) Collision With
Modelling risk of Blue Crane (Anthropoides paradiseus) collision with power lines in the Overberg region By MapuIe Kotoane Thesis presented in partial fulfilment of the requirements for the degree of Master of Arts at the University of Stellenbosch. Supervisor: Mr. A van Niekerk December 2004 Stellenbosch University http://scholar.sun.ac.za AUTHOR'S DECLARATION I, the undersigned, hereby declare that the work contained in this thesis is my original work and that I have not previously in its entirety or in part submitted it at any university for a degree. Signature: Date November 12, 2004 . 111 Stellenbosch University http://scholar.sun.ac.za ABSTRACT This study addresses the problem of Blue Crane (Anthropoides paradiseus) collisions with power lines in the Overberg region, home to approximately 50% of South Africa's national bird's global population. The low visibility of power lines against the landscape is considered to be the major cause of collisions. These claim at least 20 birds annually, which is a considerable loss to a vulnerable species. For this study, expert knowledge of the Blue Cranes' biology, general behaviour and use of its habitat were compiled. These were then translated into rules that were integrated into a Geographic Information System (GIS) to establish a predictive model, which attempts to identify and quantify risk power lines that Blue Cranes are most likely to collide with. The criteria that were considered included landscape proximity of power lines to water bodies arid congregation sites, land cover, power lines orientation in relation to predominant wind directions (North Westerly and South Easterly) and visibility of the power lines against the landscape. -

The Wolf and the Crane by Aesop
Crane Lapbook from Homeschool Share Crane Animal Study This research is provided as a simple guide. Please feel free to use your library as an additional resource for your research. Cranes are large, beautiful, graceful water birds. In many Asian cultures, cranes are a symbol of fidelity, peace, purity, wisdom, prosperity, and longevity. Use a dictionary to define these words. Add the Crane Symbols Shutterflap to your lapbook. Range Cranes are found on five of the seven continents: Africa, Asia, Australian, Europe, and North America (there are no cranes in Antarctica or South America). Complete the Where in the World Are Cranes Found? mini-book. Anatomy What Makes a Crane a Crane? Cranes come in fifteen different shapes and sizes but share these similarities: long neck long legs rounded wings pointed bill Complete the What Makes a Crane a Crane? Petal book Who’s Who in the crane family? Even though cranes have similarities, they also have differences. The tallest crane is the Sarus Crane (up to 5 3/4 feet or 1.75 m tall). The smallest Crane is the Demoiselle Crane (30 inches or 76 cm) The heaviest crane is the Red-crowned Crane (weighing up to 24 pounds or 11 kg). Complete Tallest, Smallest, Heaviest matchbooks and add to your lapbook Research one crane species and write facts on the Crane Research page, if desired. Add to pocket in your lapbook or notebook. If your student is interested in the Whooping Crane, find more information at National Geographic Kids Conservation Status Cranes are among the most vulnerable species of birds on the earth. -

Japanese Children's Books 2020 JBBY's Recommendations for Young Readers Throughout the World
JAPANESE BOARD ON BOOKS FOR YOUNG PEOPLE Japanese 2020 Children's Books 2020 Cover illustration Japanese Children's Books Chiki KIKUCHI Born in 1975 in Hokkaido. After working at a design Contents firm, he decided at age 33 to become a picture book artist. His book Shironeko kuroneko (White ● Book Selection Team ................................................................................................2 Cat, Black Cat; Gakken Plus) won a Golden Apple ● About JBBY and this Catalog ................................................................................ 3 at the 2013 Biennial of Illustrations Bratislava (BIB), and his book Momiji no tegami (Maple Leaf Letter; ● Recent Japanese Children's Books Recommended by JBBY ......................4 Komine Shoten) won a plaque at the 2019 BIB. His ● The Hans Christian Andersen Award other works include Boku da yo, boku da yo (It’s Me, Five winners and 12 nominees from Japan It’s Me; Rironsha), Chikiban nyaa (Chiki Bang Meow; ........................................................20 Gakken Plus), Pa-o-po no uta (Pa-o-po Song; Kosei ● Japanese Books Selected for the IBBY Honour List ...................................22 Shuppan), Tora no ko Torata (Torata the Tiger Cub; Children’s Literature as a Part of Japan’s Publishing Statistics ....................... Shogakukan), and Shiro to kuro (White and Black; ● Essay: 24 Kodansha). ● Recent Translations into Japanese Recommended by JBBY ....................26 JBBY Book Selection and Review Team The JBBY Book Selection and Review Team collaboratively chose the titles listed in this publication. The name in parentheses after each book description is the last name of the team member who wrote the description. Yasuko DOI Director and senior researcher at the International Insti- Yukiko HIROMATSU tute for Children’s Literature (IICLO). Besides researching Picture book author, critic, and curator. -

Hooded Crane
Hooded Crane VERTEBRATA Order: Carnivora Family: Felidae Genus: Panthera Category: 1 – critically endangered species at the territory of Russia The hooded crane (Grus monacha) is a small, dark crane, categorized as ‘vulnerable’ by IUCN. Distribution and Population: Distribution of Hooded Crane The estimated population of the species is approximately 9,200. The breeding grounds of this species are in south- eastern Siberia, Russian Federation, and northern China. More than 80 per cent of hooded cranes spend the winter at Izumi Feeding Station on the Japanese island of Kyushu. Small numbers are found at Yashiro in southern Japan (8,000 for wintering), in the Republic of Korea (100 for wintering) and the Democratic People’s Republic of Korea (100 for wintering), and at several sites along the middle Yangtze River in China (1,000 for Source: BirdLife International Species Factsheet (2013): Crus breeding and wintering). Monacha Hooded cranes nest and feed in isolated sphagnum bogs scattered through the taiga in the southeastern Russian Federation, and in China, in forested wetlands in mountain valleys. Non- breeding birds are found in shallow open wetlands, natural grasslands, and agricultural fields in southern Siberia and north-eastern Mongolia. During migration, hooded cranes often associate with Eurasian and white-naped cranes. Physical features and habitats: Adult crowns are un-feathered, red, and covered with black hair-like bristles. The head and neck are snow white, which extends down the neck. The body plumage is otherwise slaty gray. The primaries, secondaries, tail, and tail coverts are black. Juvenile crown are covered with black and white feathers during the first year, and exhibit some brownish or grayish wash on their body feathers.