Monitoring the Quality of Some Sources of Irrigation Water in Different Parts of Ogun State, Nigeria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nigeria's Constitution of 1999
PDF generated: 26 Aug 2021, 16:42 constituteproject.org Nigeria's Constitution of 1999 This complete constitution has been generated from excerpts of texts from the repository of the Comparative Constitutions Project, and distributed on constituteproject.org. constituteproject.org PDF generated: 26 Aug 2021, 16:42 Table of contents Preamble . 5 Chapter I: General Provisions . 5 Part I: Federal Republic of Nigeria . 5 Part II: Powers of the Federal Republic of Nigeria . 6 Chapter II: Fundamental Objectives and Directive Principles of State Policy . 13 Chapter III: Citizenship . 17 Chapter IV: Fundamental Rights . 20 Chapter V: The Legislature . 28 Part I: National Assembly . 28 A. Composition and Staff of National Assembly . 28 B. Procedure for Summoning and Dissolution of National Assembly . 29 C. Qualifications for Membership of National Assembly and Right of Attendance . 32 D. Elections to National Assembly . 35 E. Powers and Control over Public Funds . 36 Part II: House of Assembly of a State . 40 A. Composition and Staff of House of Assembly . 40 B. Procedure for Summoning and Dissolution of House of Assembly . 41 C. Qualification for Membership of House of Assembly and Right of Attendance . 43 D. Elections to a House of Assembly . 45 E. Powers and Control over Public Funds . 47 Chapter VI: The Executive . 50 Part I: Federal Executive . 50 A. The President of the Federation . 50 B. Establishment of Certain Federal Executive Bodies . 58 C. Public Revenue . 61 D. The Public Service of the Federation . 63 Part II: State Executive . 65 A. Governor of a State . 65 B. Establishment of Certain State Executive Bodies . -

Prof. Dr. Kayode AJAYI Dr. Muyiwa ADEYEMI Faculty of Education Olabisi Onabanjo University, Ago-Iwoye, NIGERIA
International Journal on New Trends in Education and Their Implications April, May, June 2011 Volume: 2 Issue: 2 Article: 4 ISSN 1309-6249 UNIVERSAL BASIC EDUCATION (UBE) POLICY IMPLEMENTATION IN FACILITIES PROVISION: Ogun State as a Case Study Prof. Dr. Kayode AJAYI Dr. Muyiwa ADEYEMI Faculty of Education Olabisi Onabanjo University, Ago-Iwoye, NIGERIA ABSTRACT The Universal Basic Education Programme (UBE) which encompasses primary and junior secondary education for all children (covering the first nine years of schooling), nomadic education and literacy and non-formal education in Nigeria have adopted the “collaborative/partnership approach”. In Ogun State, the UBE Act was passed into law in 2005 after that of the Federal government in 2004, hence, the demonstration of the intention to make the UBE free, compulsory and universal. The aspects of the policy which is capital intensive require the government to provide adequately for basic education in the area of organization, funding, staff development, facilities, among others. With the commencement of the scheme in 1999/2000 until-date, Ogun State, especially in the area of facility provision, has joined in the collaborative effort with the Federal government through counter-part funding to provide some facilities to schools in the State, especially at the Primary level. These facilities include textbooks (in core subjects’ areas- Mathematics, English, Social Studies and Primary Science), blocks of classrooms, furniture, laboratories/library, teachers, etc. This study attempts to assess the level of articulation by the Ogun State Government of its UBE policy within the general framework of the scheme in providing facilities to schools at the primary level. -

Prevalence, Intensity and Spatial Co-Distribution of Schistosomiasis
Parasitology Open Prevalence, intensity and spatial co-distribution of schistosomiasis and cambridge.org/pao soil transmitted helminths infections in Ogun state, Nigeria Research Article 1 1 1,2 Cite this article: Oluwole AS, Adeniran AA, Akinola S. Oluwole , Adebiyi A. Adeniran , Hammed O. Mogaji , Mogaji HO, Olabinke DB, Abe EM, Bankole SO, Dorcas B. Olabinke1, Eniola M. Abe1,3, Samuel O. Bankole1, Sam-Wobo SO, Ekpo UF (2018). Prevalence, intensity and spatial co-distribution of Sammy O. Sam-Wobo1 and Uwem F. Ekpo1 schistosomiasis and soil transmitted helminths infections in Ogun state, Nigeria. 1Department of Pure and Applied Zoology, Federal University of Agriculture, Abeokuta, Nigeria; 2Department of Parasitology Open 4, e8, 1–9. https://doi.org/ Zoology, Federal University, Oye-Ekiti, Nigeria and 3National Institute of Parasitic Diseases, Chinese Centre for 10.1017/pao.2018.4 Disease Prevention, WHO Collaborating Centre for Tropical Diseases, Shanghai, China Received: 26 March 2017 Revised: 9 February 2018 Abstract Accepted: 9 February 2018 A cross-sectional survey was carried out in primary schools to determine prevalence, intensity Key words: and spatial co-distribution of Schistosomiasis and soil transmitted helminths (STH) infections Co-distribution; prevalence; schistosomiasis; in Ogun State, Nigeria. A total of 2148 pupils from 42 schools were examined for Schistosoma soil transmitted helminths; spatial risk and STH infections from urine and fresh fecal samples respectively. Ethyl ether concentration Author for correspondence: method prepared in sodium acetate – acetic acid – formalin ether was used to concentrate Akinola S. Oluwole, E-mail: akinolaoluwole@ parasites’ ova before microscopic examination. The overall prevalence of schistosomiasis gmail.com and STH infections were 4.0% (95% CI = 3.21–4.92) and 34.64% (95% CI = 32.62–36.69) respectively. -

THR Bulletin
75 Tanzania Health Research Bulletin Vol. 8 No. 2 May 2006 MONRATE, a descriptive tool for calculation and prediction of re-infection of Ascaris lumbricoides S.O. SAM-WOBO1*, C.F. MAFIANA1, S.A. ONASHOGA2 and O.R. VINCENT2 1Department of Biological Sciences, University of Agriculture, PMB 2240, Abeokuta 110001, Ogun State, Nigeria 2Department of Computer Sciences, University of Agriculture, PMB 2240, Abeokuta 110001, Ogun State, Nigeria _______________________________________________________________________________________ Abstract: The objective of the study was to develop an interactive and systematic descriptive tool, MONRATE for calculating and predicting reinfection rates and time of Ascaris lumbricoides following mass chemotherapy using levamisole. Each pupil previously treated was retreated 6 or 7 months after the initial treatment in Ogun State, Nigeria. The implementation was based on the theoretical equation for time-prevalence: Y = G [1 -(1-X)N-R]. Using the Psuedo- Code of the MONRATE tool, the calculated monthly reinfection rates (X) for the LGAs were 1.6% in Ewekoro, 2.3% in Odeda, 2.3% in Ado-odo/Otta, 3.8% in Ogun Waterside and 4.2% in Obafemi/Owode. The mathematical mean of 'X' values in the study areas for Ogun State was 2.84. The calculated reinfection time (N months) for the LGAs varied such as Ado-odo/Otta (12.7), Ogun Waterside (21.8), Obafemi/Owode (22.92), Odeda (25.45), and Ewekoro (25.9). The mean value for N in Ogun State was 21.75. The results obtained from MONRATE were compared with those obtained using the mathematical equation and were found to be the same but MONRATE was faster in computation and more accurate. -

A Case Study in Ikenne Local Government, Ogun State, Nigeria
Quest Journals Journal of Research in Agriculture and Animal Science Volume 3 ~ Issue 10 (2016) pp:07-13 ISSN(Online) : 2321-9459 www.questjournals.org Research Paper Determinants of Crop Farmers’ Adoption of Soil Conservation Techniques: A Case Study in Ikenne Local Government, Ogun State, Nigeria. Bello Taofeek Ayodeji1, *Afodu Osagie John1, Ndubuisi-Ogbonna Lois Chidinma2, Akinboye Olufunso Emmanuel3 Akpabio, Utibe-Obong Enobong1 1Department of Agricultural Economics & Extension, 2Department of Animal Science, 3Department of Agronomy and Landscape Design, School of Agriculture and Industrial Technology, Babcock University, Ilishan Remo, Ogun state, Nigeria. Received 06 February, 2016; Accepted 16 March, 2016 © The author(s) 2015. Published with open access at www.questjournals.org ABSTRACT:- Soil conservation is a set of management strategies for prevention of soil being eroded from the earth’s surface or becoming chemically altered by overuse, salinization acidification, or other chemical soil contamination. Soil conservation technique is the application of processes to the solution of soil management problems. This research assessed the level of crop farmers’ awareness of soil conservation, described the socio- economic characteristics of the crop farmers, and evaluated factors that determine or influence their adoption of soil conservation techniques in Ikenne local government area of Ogun State. One hundred (100) crop farmers were selected randomly for the research study but out of all the 100 questionnaires administered, only 97 were found useful for analysis. The demographic data collected were analysed using descriptive statistics, while the logit regression model was used to evaluate the factors determining crop farmers’ adoption of soil conservation techniques. The descriptive analysis result showed that 61.9% of the respondents had farming as their major occupation, 87.6% had farmlands of their own, 38.1% belonged to farmers’ groups/associations, and 71.1% were aware of soil conservation techniques. -

Odo/Ota Local Government Secretariat, Sango - Agric
S/NO PLACEMENT DEPARTMENT ADO - ODO/OTA LOCAL GOVERNMENT SECRETARIAT, SANGO - AGRIC. & BIO. ENGINEERING 1 OTA, OGUN STATE AGEGE LOCAL GOVERNMENT, BALOGUN STREET, MATERNITY, AGRIC. & BIO. ENGINEERING 2 SANGO, AGEGE, LAGOS STATE AHMAD AL-IMAM NIG. LTD., NO 27, ZULU GAMBARI RD., ILORIN AGRIC. & BIO. ENGINEERING 3 4 AKTEM TECHNOLOGY, ILORIN, KWARA STATE AGRIC. & BIO. ENGINEERING 5 ALLAMIT NIG. LTD., IBADAN, OYO STATE AGRIC. & BIO. ENGINEERING 6 AMOULA VENTURES LTD., IKEJA, LAGOS STATE AGRIC. & BIO. ENGINEERING CALVERTON HELICOPTERS, 2, PRINCE KAYODE, AKINGBADE MECHANICAL ENGINEERING 7 CLOSE, VICTORIA ISLAND, LAGOS STATE CHI-FARM LTD., KM 20, IBADAN/LAGOS EXPRESSWAY, AJANLA, AGRIC. & BIO. ENGINEERING 8 IBADAN, OYO STATE CHINA CIVIL ENGINEERING CONSTRUCTION CORPORATION (CCECC), KM 3, ABEOKUTA/LAGOS EXPRESSWAY, OLOMO - ORE, AGRIC. & BIO. ENGINEERING 9 OGUN STATE COCOA RESEARCH INSTITUTE OF NIGERIA (CRIN), KM 14, IJEBU AGRIC. & BIO. ENGINEERING 10 ODE ROAD, IDI - AYANRE, IBADAN, OYO STATE COKER AGUDA LOCAL COUNCIL, 19/29, THOMAS ANIMASAUN AGRIC. & BIO. ENGINEERING 11 STREET, AGUDA, SURULERE, LAGOS STATE CYBERSPACE NETWORK LTD.,33 SAKA TIINUBU STREET. AGRIC. & BIO. ENGINEERING 12 VICTORIA ISLAND, LAGOS STATE DE KOOLAR NIGERIA LTD.,PLOT 14, HAKEEM BALOGUN STREET, AGRIC. & BIO. ENGINEERING OPP. TECHNICAL COLLEGE, AGIDINGBI, IKEJA, LAGOS STATE 13 DEPARTMENT OF PETROLEUM RESOURCES, 11, NUPE ROAD, OFF AGRIC. & BIO. ENGINEERING 14 AHMAN PATEGI ROAD, G.R.A, ILORIN, KWARA STATE DOLIGERIA BIOSYSTEMS NIGERIA LTD, 1, AFFAN COMPLEX, 1, AGRIC. & BIO. ENGINEERING 15 OLD JEBBA ROAD, ILORIN, KWARA STATE Page 1 SIWES PLACEMENT COMPANIES & ADDRESSES.xlsx S/NO PLACEMENT DEPARTMENT ESFOOS STEEL CONSTRUCTION COMPANY, OPP. SDP, OLD IFE AGRIC. & BIO. ENGINEERING 16 ROAD, AKINFENWA, EGBEDA, IBADAN, OYO STATE 17 FABIS FARMS NIGERIA LTD., ILORIN, KWARA STATE AGRIC. -

In Abeokuta North Local Government, Nigeria
l o rna f Wa ou s OPEN ACCESS Freely available online J te l a R n e o s i o t u a International Journal of r n c r e e t s n I ISSN: 2252-5211 Waste Resources Research Article Water Quality Assessment of Groundwater (Hand-Dug Wells) in Abeokuta North Local Government, Nigeria Falola TO1*, Adetoro IO and Idowu OA2 1Student at Federal University of Technology, Akure, Nigeria; 2Nigeria Department of Biological Sciences, University of Agriculture, Abeokuta, Nigeria ABSTRACT Groundwater is the major source of water for municipal use in the AbeokutaNorth Local Government of Nigeria. However, there is a tendency for its quality to deviate from recommended standards as most groundwater sources are close to regions prone to erosion and most well are not usually covered. The tragedy is that the adverse effect might creep into the ecosystem and affects humanity if a regular check on the quality is not been made. The Geographical location and altitude of each well location were taken using Global Positioning System (GPS). The moderate PH range (6.30-7.36) can be linked to low values of TDS (352 mg/L) and EC (695 ms/cm) which are within the standard recommended for drinking- indicate a low concentration of salt contents. The relationship between the parameters shows a direct trend with the hydraulic head. Hence, groundwater sources (wells) in Abeokuta North Local Government are good for drinking. Keywords: Groundwater; Abeokuta North; Global Positioning System; PH; Total DissolvedSolid; Electrical conductivity; Hydraulic head; W.H.O INTRODUCTION Water quality describes the physical, chemical, and microbiological characteristics of water. -

Socio-Economic Factors of Cooperative Farmer's and Their Food Intake in Yewa North Local Government Area of Ogun State
Acta Scientific AGRICULTURE (ISSN: 2581-365X) Volume 4 Issue 8 August 2020 Research Article Socio-Economic Factors of Cooperative Farmer’s and their Food Intake in Yewa North Local Government Area of Ogun State Oluwasanya OP1*, Nwankwo FO2, Aladegoroye OR1 and Ojewande AA3 Received: March 31, 2020 1Department of Cooperative and Rural Development, Olabisi Onabanjo University, Published: July 30, 2020 Ago-Iwoye, Ogun State, Nigeria © All rights are reserved by Oluwasanya OP., 2Department of Cooperative Economics and Management, Nnamdi Azikiwe University, et al. Awka, Nigeria 3Department of Banking and Finance, Osun State University, Osogbo, Nigeria *Corresponding Author: Oluwasanya OP, Department of Cooperative and Rural Development, Olabisi Onabanjo University, Ago-Iwoye, Ogun State, Nigeria. Abstract The study analysed effect of socio-economic characteristics of cooperative farmers’ on their food intake in Yewa North Local Gov- ernment Area, Ogun State with a view to providing policy information toward enhancing the nutritional status of Nigeria. Hunger and malnutrition in developing countries like Nigeria requires the improvement of goals to lower the rate of frequently malnourished individual. There is problem of food and nutrition security in the world today. The data was collected through multistage sampling to obtain useful data from 112 households. It was revealed that 76.8% of the household farmers had average income below N30,000 per month. The household farmer’s expenditure was N4,961.24 and per capital average expenditure was N925.605. This showed that poverty level is very critical and needs urgent attention in the study area. On this note, it was recommended that appropriate, attainable and practicable programme should be done to alleviate poverty and enhance income among rural farmers and that there Nigerians most especially food insecure and vulnerable individual. -

Nigeria's Constitution of 1999
PDF generated: 14 Apr 2014, 20:49 constituteproject.org Nigeria's Constitution of 1999 This complete constitution has been generated from excerpts of texts from the repository of the Comparative Constitutions Project, and distributed on constituteproject.org. constituteproject.org PDF generated: 14 Apr 2014, 20:49 Preamble We the people of the Federal Republic of Nigeria Having firmly and solemnly resolved, to live in unity and harmony as one indivisible and indissoluble sovereign nation under God, dedicated to the promotion of inter-African solidarity, world peace, international co- operation and understanding And to provide for a Constitution for the purpose of promoting the good government and welfare of all persons in our country, on the principles of freedom, equality and justice, and for the purpose of consolidating the unity of our people Do hereby make, enact and give to ourselves the following Constitution:- Chapter I General Provisions Part I Federal Republic of Nigeria 1. 1. This Constitution is supreme and its provisions shall have binding force on the authorities and persons throughout the Federal Republic of Nigeria. 2. The Federal Republic of Nigeria shall not be governed, nor shall any persons or group of persons take control of the Government of Nigeria or any part thereof, except in accordance with the provisions of this Constitution. 3. If any other law is inconsistent with the provisions of this Constitution, this Constitution shall prevail, and that other law shall, to the extent of the inconsistency, be void. 2. 1. Nigeria is one indivisible and indissoluble sovereign state to be known by the name of the Federal Republic of Nigeria. -
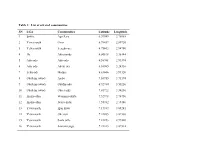
Table 1: List of Selected Communities SN LGA Communities Latitude
Table 1: List of selected communities SN LGA Communities Latitude Longitude 1 Ipokia Ago Sasa 6.59089 2.76065 2 Yewa-south Owo 6.78457 2.89720 3 Yewa-south Ireagbo-are 6.75602 2.94780 4 Ifo Akinsinnde 6.80818 3.16144 5 Ado-odo Ado-odo 6.58768 2.93374 6 Ado-odo Abebi-ota 6.68965 3.24330 7 Ijebu-ode Molipa 6.83606 3.91120 8 Obafemi-owode Ajebo 7.10955 3.71174 9 Obafemi-owode Odofin-odo 6.92744 3.55220 10 Obafemi-owode Oba-seriki 7.01712 3.34230 11 Imeko-afon Wasinmi-okuta 7.52948 2.76750 12 Imeko-afon Iwoye-ketu 7.55782 2.74486 13 Yewa-north Igan ikoto 7.15339 3.04281 14 Yewa-north Oke rori 7.24805 3.02368 15 Yewa-north Saala orile 7.21253 2.97420 16 Yewa-north Araromi joga 7.23323 3.02514 17 Ewekoro Abule Oko 6.86859 3.19430 18 Shagamu Ipoji 6.84440 3.65006 19 Shagamu Odelemo 6.74479 3.66392 20 Ikenne Irolu 6.90834 3.72447 21 Odogbolu Ikosa 6.83873 3.76291 22 Ijebu-east Itele 6.76299 4.06629 23 Ijebu-east Imobi 6.65920 4.17934 24 Ijebu north-east Atan 6.89712 4.00414 25 Abeokuta-south Ibon 7.15864 3.35519 26 Ijebu north Agric 6.93907 3.83253 27 Ijebu north Japara 6.97274 3.99278 28 Remo north Akaka 6.94053 3.71328 29 Odeda Alabata 7.31567 3.53351 30 Odeda Olodo 7.29659 3.60758 31 Abeokuta north Imala odo 7.32122 3.18115 32 Ogun water-side Abigi 6.48618 4.39408 33 Ogun water-side Iwopin 6.51054 4.16990 Table 2: Sex and age distribution of study participants SN LGA Sex (%) Age in years (%) Number Male Female <5yrs 5-15yrs 16-25yrs 26-40yrs 41-70yrs >70yrs Examined 1 Abeokuta north 87 28(32.2) 59(67.8) 7(8.0) 64(7.6) 9(10.3) 3(3.4) 4(4.6) -

Ethnic Minorities and Land Conflicts in Southwestern Nigeria
SOCIAL SCIENCE RESEARCH COUNCIL | WORKING PAPERS ETHNIC MINORITIES AND LAND CONFLICTS IN SOUTHWESTERN NIGERIA JEREMIAH O. AROWOSEGBE AFRICAN PEACEBUILDING NETWORK APN WORKING PAPERS: NO. 14 This work carries a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License. This license permits you to copy, distribute, and display this work as long as you mention and link back to the Social Science Research Council, attribute the work appropriately (including both author and title), and do not adapt the content or use it commercially. For details, visit http://creativecommons.org/licenses/by-nc-nd/3.0/us/. ABOUT THE PROGRAM Launched in March 2012, the African Peacebuilding Network (APN) supports independent African research on conflict-affected countries and neighboring regions of the continent, as well as the integration of high-quality African research-based knowledge into global policy communities. In order to advance African debates on peacebuilding and promote African perspectives, the APN offers competitive research grants and fellowships, and it funds other forms of targeted support, including strategy meetings, seminars, grantee workshops, commissioned studies, and the publication and dissemination of research findings. In doing so, the APN also promotes the visibility of African peacebuilding knowledge among global and regional centers of scholarly analysis and practical action and makes it accessible to key policymakers at the United Nations and other multilateral, regional, and national policymaking institutions. ABOUT THE SERIES “African solutions to African problems” is a favorite mantra of the African Union, but since the 2002 establishment of the African Peace and Security Architecture, the continent has continued to face political, material, and knowledge-related challenges to building sustainable peace. -

Spatial Distribution of Ascariasis, Trichuriasis and Hookworm Infections in Ogun State, Southwestern Nigeria
Spatial Distribution of Ascariasis, Trichuriasis and Hookworm Infections in Ogun State, Southwestern Nigeria Hammed Mogaji ( [email protected] ) Federal University Oye-Ekiti https://orcid.org/0000-0001-7330-2892 Gabriel Adewunmi Dedeke Federal University of Agriculture Abeokuta Babatunde Saheed Bada Federal University of Agriculture Abeokuta Samuel O. Bankole Federal University of Agriculture Abeokuta Adejuwon Adeniji Federal University of Agriculture Abeokuta Mariam Tobi Fagbenro Federal University of Agriculture Abeokuta Olaitan Olamide Omitola Federal University of Agriculture Abeokuta Akinola Stephen Oluwole SightSavers Nnayere Simon Odoemene Adeleke University Uwem Friday Ekpo Federal University of Agriculture Abeokuta Research article Keywords: Spatial Mapping, Distribution, Ascariasis, Trichuriasis, Hookworm, Ogun State, Nigeria Posted Date: July 29th, 2019 DOI: https://doi.org/10.21203/rs.2.12035/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/20 Abstract Background Ascariasis, Trichuriasis and Hookworm infections poses a considerable public health burden in Sub-Saharan Africa, and a sound understanding of their spatial distribution facilitates to better target control interventions. This study, therefore, assessed the prevalence of the trio, and mapped their spatial distribution in the 20 administrative regions of Ogun State, Nigeria. Methods Parasitological surveys were carried out in 1,499 households across 33 spatially selected communities. Fresh stool samples were collected from 1,027 consenting participants and processed using ether concentration method. Households were georeferenced using a GPS device while demographic data were obtained using a standardized form. Data were analysed using SPSS software and visualizations and plotting maps were made in ArcGIS software. Results Findings showed that 19 of the 20 regions were endemic for one or more kind of the three infections, with an aggregated prevalence of 17.2%.